Abstract
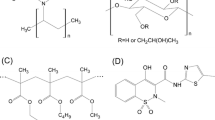
In the current work, screening of polymers viz. polyacrylic acid (PAA), polyvinyl pyrrolidone vinyl acetate (PVP VA), and hydroxypropyl methyl cellulose acetate succinate (HPMC AS) based on drug-polymer interaction and wetting property was done for the production of a stable amorphous solid dispersion (ASD) of a poorly water-soluble drug Riluzole (RLZ). PAA showed maximum interaction and wetting property hence, was selected for further studies. Solid state characterization studies confirmed the formation of ASD with PAA. Saturation solubility, dissolution profile, and in vivo pharmacokinetic data of the ASD formulation were generated in rats against its marketed tablet Rilutor. The RLZ:PAA ASD showed exponential enhancement in the dissolution of RLZ. Predicted and observed pharmacokinetic data in rats showed enhanced area under curve (AUC) and Cmax in plasma and brain with respect to Rilutor. Furthermore, a physiologically based pharmacokinetic (PBPK) model of rats for Rilutor and RLZ ASD was developed and then extrapolated to humans where physiological parameters were changed along with a biochemical parameter. The partition coefficient was kept similar in both species. The model was used to predict different exposure scenarios, and the simulated data was compared with observed data points. The PBPK model simulated Cmax and AUC was within two times the experimental data for plasma and brain. The Cmax and AUC in the brain increased with ASD compared to Rilutor for humans showing its potential in improving its biopharmaceutical performance and hence enhanced therapeutic efficacy. The model can predict the RLZ concentration in multiple compartments including plasma and liver.
Graphical Abstract










Similar content being viewed by others
Data Availability
The experimental data can be made available upon request. The model code will be uploaded to GITHUB and also facilitated on Rshiny server and can be provided upon request.
References
Amponsah-Efah KK, Mistry P, Eisenhart R, Suryanarayanan R. The influence of the strength of drug–polymer interactions on the dissolution of amorphous solid dispersions. Mol Pharm. 2020;18(1):174–86. https://doi.org/10.1021/acs.molpharmaceut.0c00790.
Bhujbal SV, Mitra B, Jain U, Gong Y, Agrawal A, Karki S, Taylor LS, Kumar S, Zhou QT. Pharmaceutical amorphous solid dispersion: a review of manufacturing strategies. Acta Pharm Sin B. 2021;11(8):2505–36. https://doi.org/10.1016/j.apsb.2021.05.014.
Dhondale MR, Thakor P, Nambiar AG, Singh M, Agrawal AK, Shastri NR, Kumar D. Co-Crystallization approach to enhance the stability of moisture-sensitive drugs. Pharmaceutics. 2023;15(1):189. https://doi.org/10.3390/pharmaceutics15010189.
Dash RP, Babu RJ, Srinivas NR. Two decades-long journey from riluzole to edaravone: revisiting the clinical pharmacokinetics of the only two amyotrophic lateral sclerosis therapeutics. Clin Pharmacokinet. 2018;57:1385–98. https://doi.org/10.1007/s40262-018-0655-4.
Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47(6 Suppl 4):233S-S241. https://doi.org/10.1212/WNL.47.6_Suppl_4.233S.
Dharmadasa T, Kiernan MC. Riluzole, disease stage and survival in ALS. Lancet Neurol. 2018;17(5):385–6. https://doi.org/10.1016/S1474-4422(18)30091-7.
Lacomblez L, Bensimon G, Leigh PN, Debove C, Bejuit R, Truffinet P, Meininger V. Long-term safety of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders. 2002;3(1):23–9. https://doi.org/10.1080/146608202317576507.
Bensimon G, Doble A. The tolerability of riluzole in the treatment of patients with amyotrophic lateral sclerosis. Expert opinion on drug safety. 2004;3(6):525–34. https://doi.org/10.1517/14740338.3.6.525.
Brouwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J Pharm Sci. 2009;98(8):2549–72. https://doi.org/10.1002/jps.21650.
Tsai YM, Chien CF, Lin LC, Tsai TH. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood–brain barrier penetration. Int J Pharm. 2011;416(1):331–8. https://doi.org/10.1016/j.ijpharm.2011.06.030.
Deepika D, Kumar V. The role of “physiologically based pharmacokinetic model (PBPK)” new approach methodology (NAM) in pharmaceuticals and environmental chemical risk assessment. Int J Environ Res Public Health. 2023;20(4):3473. https://doi.org/10.3390/ijerph20043473.
Van Kan HJ, Spieksma M, Groeneveld GJ, Toraño JS, Van den Berg LH, Guchelaar HJ. A validated HPLC assay to monitor riluzole plasma or serum concentrations in patients with amyotrophic lateral sclerosis. Biomed Chromatogr. 2004;18(9):723–6. https://doi.org/10.1002/bmc.384.
Ravi PR, Vats R, Reddy KU. Validation of a simple, rapid and sensitive LC method for quantification of riluzole in rat plasma and its pharmacokinetic application. J Bioanal Biomed S. 2012;6:2. https://doi.org/10.4172/1948-593X.S6-007.
Rohatgi A. WebPlotDigitizer (Version 3.9) [Computer software]. 2015. Available from: https://automeris.io/WebPlotDigitizer.
Liboux AL, Lefebvre P, Roux YL, Truffinet P, Aubeneau M, Kirkesseli S, Montay G. Single-and multiple-dose pharmacokinetics of riluzole in white subjects. J Clin Pharmacol. 1997;37(9):820–7. https://doi.org/10.1002/j.1552-4604.1997.tb05630.x.
Chandu BR, Nama S, Kanala K, Challa BR, Shaik RP, Khagga M. Quantitative estimation of riluzole in human plasma by LC-ESI-MS/MS and its application to a bioequivalence study. Anal Bioanal Chem. 2010;398:1367–74. https://doi.org/10.1007/s00216-010-4034-8.
Longo DM, Shoda LK, Howell BA, Coric V, Berman RM, Qureshi IA. Assessing effects of BHV-0223 40 mg Zydis sublingual formulation and Riluzole 50 mg Oral tablet on liver function test parameters utilizing DILIsym. Toxicol Sci. 2020;175(2):292–300. https://doi.org/10.1093/toxsci/kfaa019.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57. https://doi.org/10.1002/jps.20502.
Utsey K, Gastonguay MS, Russell S, Freling R, Riggs MM, Elmokadem A. Quantification of the impact of partition coefficient prediction methods on physiologically based pharmacokinetic model output using a standardized tissue composition. Drug Metab Dispos. 2020;48(10):903–16. https://doi.org/10.1124/dmd.120.090498.
Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, Chang Z, Woolsey J. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006;34(Database issue):D668–72. https://doi.org/10.1093/nar/gkj067.
Hu ZY, Lu J, Zhao Y. A physiologically based pharmacokinetic model of alvespimycin in mice and extrapolation to rats and humans. Br J Pharmacol. 2014;171(11):2778–89. https://doi.org/10.1111/bph.12609.
Campbell JL Jr, Andersen ME, Hinderliter PM, Yi KD, Pastoor TP, Breckenridge CB, Clewell HJ III. PBPK model for atrazine and its chlorotriazine metabolites in rat and human. Toxicol Sci. 2016;150(2):441–53. https://doi.org/10.1093/toxsci/kfw014.
Stevens AJ, Campbell Jr JL, Travis KZ, Clewell III HJ, Hinderliter PM, Botham PA, Cook AR, Minnema DJ, Wolf DC. Paraquat pharmacokinetics in primates and extrapolation to humans. Toxicol ApplPharmacol. 2021;417:115463. https://doi.org/10.1016/j.taap.2021.115463.
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health. 1997;13(4):407–84. https://doi.org/10.1177/074823379701300401.
Emond C, Raymer JH, Studabaker WB, Garner CE, Birnbaum LS. A physiologically based pharmacokinetic model for developmental exposure to BDE-47 in rats. Toxicol Appl Pharmacol. 2010;242(3):290–8. https://doi.org/10.1016/j.taap.2009.10.019.
Sharma RP, Kumar V, Schuhmacher M, Kolodkin A, Westerhoff HV. Development and evaluation of a harmonized whole body physiologically based pharmacokinetic (PBPK) model for flutamide in rats and its extrapolation to humans. Environ Res. 2020;182:108948. https://doi.org/10.1016/j.envres.2019.108948.
Loccisano AE, Campbell Jr JL, Andersen ME, Clewell III HJ. Evaluation and prediction of pharmacokinetics of PFOA and PFOS in the monkey and human using a PBPK model. Regul Toxicol Pharmacol. 2011;59(1):157–75. https://doi.org/10.1016/j.yrtph.2010.12.004.
Shah DK, Betts AM. Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn. 2012;39:67–86. https://doi.org/10.1007/s10928-011-9232-2.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093–5. https://doi.org/10.1023/A:1018943613122.
Milane A, Tortolano L, Fernandez C, Bensimon G, Meininger V, Farinotti R. Brain and plasma riluzole pharmacokinetics: effect of minocycline combination. J Pharm Pharm Sci. 2009;12(2):209–17. https://doi.org/10.18433/J36C78.
Saboo S, Moseson DE, Kestur US, Taylor LS. Patterns of drug release as a function of drug loading from amorphous solid dispersions: a comparison of five different polymers. Eur J Pharm Sci. 2020;1(155): 105514. https://doi.org/10.1016/j.ejps.2020.105514.
Patel RJ, Patel AA, Patel HP. Stabilized amorphous state of riluzole by immersion-rotavapor method with synthesized mesoporous SBA-15 carrier to augment in-vitro dissolution. J Drug Deliv Sci Technol. 2021;61:102270. https://doi.org/10.18433/J36C78.
Jasani MS, Kale DP, Singh IP, Bansal AK. Influence of drug–polymer interactions on dissolution of thermodynamically highly unstable cocrystal. Mol Pharm. 2018;16(1):151–64. https://doi.org/10.1021/acs.molpharmaceut.8b00923.
Baghel S, Cathcart H, O’Reilly NJ. Understanding the generation and maintenance of supersaturation during the dissolution of amorphous solid dispersions using modulated DSC and 1H NMR. Int J Pharm. 2018;536(1):414–25. https://doi.org/10.1016/j.ijpharm.2017.11.056.
Ueda K, Higashi K, Moribe K. Unusual correlation between the apparent amorphous solubility of a drug and solubilizer concentration revealed by NMR analysis. Mol Pharm. 2022;19(9):3336–49. https://doi.org/10.1021/acs.molpharmaceut.2c00478.
Abraham RJ, Mobli M. An NMR, IR and theoretical investigation of 1H chemical shifts and hydrogen bonding in phenols. Magn Reson Chem. 2007;45(10):865–77. https://doi.org/10.1002/mrc.2060.
Yu D, Fiddler F, Ibrahim A, Sanedrin R, Tremblay H, Hoag SW. Surface characterization as a tool for identifying the factors affecting the dissolution rate of amorphous solid dispersion tablets. AAPS PharmSciTech. 2022;23(8):282. https://doi.org/10.1208/s12249-022-02413-7.
Zenoozi S, Sadeghi GM, Rafiee M. Synthesis and characterization of biocompatible semi-interpenetrating polymer networks based on polyurethane and cross-linked poly (acrylic acid). Eur Polymer J. 2020;5(140): 109974. https://doi.org/10.1016/j.eurpolymj.2020.109974.
S’ari M, Blade H, Cosgrove S, Drummond-Brydson R, Hondow N, Hughes LP, Brown A. Characterization of amorphous solid dispersions and identification of low levels of crystallinity by transmission electron microscopy. Mol Pharm. 2021;18(5):1905–19. https://doi.org/10.1021/acs.molpharmaceut.0c00918.
Marsac PJ, Rumondor AC, Nivens DE, Kestur US, Stanciu L, Taylor LS. Effect of temperature and moisture on the miscibility of amorphous dispersions of felodipine and poly (vinyl pyrrolidone). J Pharm Sci. 2010;99(1):169–85. https://doi.org/10.1002/jps.21809.
Narasimham L, Barhate VD. Physico-chemical characterization of some beta blockers and anti-diabetic drugs-potentiometric and spectrophotometric pKa determination in different co-solvents. Eur J Chem. 2011;2(1):36–46. https://doi.org/10.5155/eurjchem.2.1.36-46.371.
Fehlings MG, Wilson JR, Frankowski RF, Toups EG, Aarabi B, Harrop JS, Shaffrey CI, Harkema SJ, Guest JD, Tator CH, Burau KD. Riluzole for the treatment of acute traumatic spinal cord injury: rationale for and design of the NACTN Phase I clinical trial. J Neurosurg Spine. 2012;17(Suppl1):151–6. https://doi.org/10.3171/2012.4.AOSPINE1259.
Correa-Soto CE, Gao Y, Indulkar AS, Zhang GG, Taylor LS. Role of surfactants in improving release from higher drug loading amorphous solid dispersions. Int J Pharm. 2022;25(625): 122120. https://doi.org/10.1016/j.ijpharm.2022.122120.
Mah PT, Peltonen L, Novakovic D, Rades T, Strachan CJ, Laaksonen T. The effect of surfactants on the dissolution behavior of amorphous formulations. Eur J Pharm Biopharm. 2016;1(103):13–22. https://doi.org/10.1016/j.ejpb.2016.03.007.
Que C. Optimization of dissolution performance for amorphous solid dispersions. Purdue University Graduate School. Thesis. 2019. https://doi.org/10.25394/PGS.11301389.v1.
Indulkar AS, Lou X, Zhang GG, Taylor LS. Insights into the dissolution mechanism of ritonavir–copovidone amorphous solid dispersions: importance of congruent release for enhanced performance. Mol Pharm. 2019;16(3):1327–39. https://doi.org/10.1021/acs.molpharmaceut.8b01261.
Saboo S, Mugheirbi NA, Zemlyanov DY, Kestur US, Taylor LS. Congruent release of drug and polymer: a “sweet spot” in the dissolution of amorphous solid dispersions. J Control Release. 2019;28(298):68–82. https://doi.org/10.1016/j.jconrel.2019.01.039.
Yang R, Zhang GG, Zemlyanov DY, Purohit HS, Taylor LS. Release mechanisms of amorphous solid dispersions: role of drug-polymer phase separation and morphology. J Pharm Sci. 2023;112(1):304–17. https://doi.org/10.1016/j.xphs.2022.10.021.
Mistry P, Mohapatra S, Gopinath T, Vogt FG, Suryanarayanan R. Role of the strength of drug–polymer interactions on the molecular mobility and crystallization inhibition in ketoconazole solid dispersions. Mol Pharm. 2015;12(9):3339–50. https://doi.org/10.1021/acs.molpharmaceut.5b00333.
Thakore SD, Thakur PS, Shete G, Gangwal R, Narang AS, Sangamwar AT, Bansal AK. Assessment of biopharmaceutical performance of supersaturating formulations of carbamazepine in rats using physiologically based pharmacokinetic modeling. AAPS PharmSciTech. 2019;20:1–2. https://doi.org/10.1208/s12249-019-1386-z.
Deepika D, Sharma RP, Schuhmacher M, Kumar V. Risk assessment of perfluorooctane sulfonate (PFOS) using dynamic age dependent physiologically based pharmacokinetic model (PBPK) across human lifetime. Environ Res. 2021;1(199): 111287. https://doi.org/10.1016/j.envres.2021.111287.
Choi GW, Lee YB, Cho HY. Interpretation of non-clinical data for prediction of human pharmacokinetic parameters: in vitro-in vivo extrapolation and allometric scaling. Pharmaceutics. 2019;11(4):168. https://doi.org/10.3390/pharmaceutics11040168.
Remy AJ, Camu W, Ramos J, Blanc P, Larrey D. Acute hepatitis after riluzole administration. J Hepatol. 1999;30(3):527–30. https://doi.org/10.1016/S0168-8278(99)80115-9.
Sheng YJ, Wu G, He HY, Chen W, Zou YS, Li Q, Zhong L, Huang YM, Deng CL. The association between CYP2E1 polymorphisms and hepatotoxicity due to anti-tuberculosis drugs: a meta-analysis. Infect Genet Evol. 2014;1(24):34–40. https://doi.org/10.1016/j.meegid.2014.01.034.
Purohit HS, Trasi NS, Sun DD, Chow EC, Wen H, Zhang X, Gao Y, Taylor LS. Investigating the impact of drug crystallinity in amorphous tacrolimus capsules on pharmacokinetics and bioequivalence using discriminatory in vitro dissolution testing and physiologically based pharmacokinetic modeling and simulation. J Pharm Sci. 2018;107(5):1330–41. https://doi.org/10.1016/j.xphs.2017.12.024.
Author information
Authors and Affiliations
Contributions
Kanchan Bharti: conceptualization, methodology, formal analysis, investigation, data curation, writing — original draft, visualization, project administration. Deepika: methodology, software, validation, formal analysis, investigation, data curation, writing — original draft, visualization. Manish Kumar: methodology, investigation, data curation. Abhishek Jha: methodology, investigation, data curation. Manjit: methodology, investigation, data curation. Akhilesh: methodology, investigation, data curation. Vinod Tiwari: resources, data interpretation, writing — original draft. Vikas Kumar: resources, data interpretation, writing — original draft. Brahmeshwar Mishra: conceptualization, resources, supervision, visualization, project administration, writing — original draft and final editing.
Corresponding author
Ethics declarations
Ethics Approval
The ethical approval for conducting animal experiment was taken from the committee (IIT(BHU)/IAEC/2022/010).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bharti, K., Deepika, D., Kumar, M. et al. Development and Evaluation of Amorphous Solid Dispersion of Riluzole with PBPK Model to Simulate the Pharmacokinetic Profile. AAPS PharmSciTech 24, 219 (2023). https://doi.org/10.1208/s12249-023-02680-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02680-y