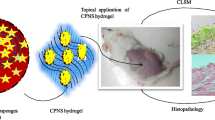
Abstract
Psoriasis is an auto-immune condition with high keratinocyte hyperproliferation due to lower p53 and p22 levels. Tacrolimus, an immune suppressor, is considered one of the most effective drugs in suppressing psoriasis. Systematic administration of tacrolimus often leads to challenging side effects, namely increased infection risk, renal toxicity, neurological symptoms such as tremors and headaches, gastrointestinal disturbances, hypertension, skin-related problems, etc. To address this, a nanocarrier-based formulation of tacrolimus along with inclusion of hyaluronic acid was developed. The optimization and formulation of ethosomes via the ethanol injection technique were done based on the Box-Behnken experimental design. The results revealed hyaluronic acid–based tacrolimus ethosomes (HA-TAC-ETH) had nanometric vesicle size (315.7 ± 2.2 nm), polydispersity index (PDI) (0.472 ± 0.07), and high entrapment efficiency (88.3 ± 2.52%). The findings of drug release and skin permeation showed sustained drug release with increased dermal flux and enhancement ratio. The effectiveness of HA-TAC-ETH was confirmed in an imiquimod (5%)–prompted psoriasis model. The skin irritation score and Psoriasis Area and Severity Index (PASI) score indicated that HA-TAC-ETH gel has validated a decline in the entire factors (erythema, edema, and thickness) in the imiquimod-induced psoriasis model in contrast with TAC-ETH gel and TAC ointment. The fabricated HA-TAC-ETH opt gel proved to be safe and effective in in vivo studies and could be employed to treat psoriasis further.







Similar content being viewed by others
Abbreviations
- TAC:
-
Tacrolimus
- ETH:
-
Ethosomes
- HA:
-
Hyaluronic acid
- BBD:
-
Box-Behnken Design
References
Deng Y, Chang C, Lu Q. The inflammatory response in psoriasis: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:377–89.
Lønnberg AS, Skov L. Co-morbidity in psoriasis: mechanisms and implications for treatment. Expert Rev Clin Immunol. 2017;13(1):27–34.
Ghosh D, Ganguly T, Chatterjee R. Emerging roles of non-coding RNAs in psoriasis pathogenesis. Funct Integr Genom. 2023;23(2):129.
Fleming P, Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, et al. Effect of biologics on depressive symptoms in patients with psoriasis: a systematic review. J Eur Acad Dermatol Venereol. 2015;29(6):1063–70.
de Morales JMGR, Puig L, Daudén E, Cañete JD, Pablos JL, Martín AO, et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: an updated review of the evidence focusing in controversies. Autoimmun Rev. 2020;19(1): 102429.
Nordin UU, Ahmad N, Salim N, Yusof NS. Lipid-based nanoparticles for psoriasis treatment: a review on conventional treatments, recent works, and future prospects. RSC Adv. 2021;11(46):29080–101.
Yamanaka K, Yamamoto O, Honda T. Pathophysiology of psoriasis: a review. J Dermatol. 2021;48(6):722–31.
Blauvelt A. New concepts in the pathogenesis and treatment of psoriasis: key roles for IL-23, IL-17A and TGF-β1. Expert Rev Dermatol. 2007;2(1):69–78.
Kamata M, Tada Y. Safety of biologics in psoriasis. J Dermatol. 2018;45(3):279–86.
Jafari A, Daneshamouz S, Ghasemiyeh P, Mohammadi-Samani S. Ethosomes as dermal/transdermal drug delivery systems: applications, preparation and characterization. J Liposome Res. 2023;33(1):34–52.
Garg V, Singh H, Bimbrawh S, Kumar Singh S, Gulati M, Vaidya Y, et al. Ethosomes and transfersomes: principles, perspectives and practices. Curr Drug Deliv. 2017;14(5):613–33.
Pandey V, Golhani D, Shukla R. Ethosomes: versatile vesicular carriers for efficient transdermal delivery of therapeutic agents. Drug Deliv. 2015;22(8):988–1002.
Arcas JM, González A, Gers-Barlag K, González-González O, Bech F, Demirkhanyan L, et al. The immunosuppressant macrolide tacrolimus activates cold-sensing TRPM8 channels. J Neurosci. 2019;39(6):949–69.
De Gregori S, De Silvestri A, Cattadori B, Rapagnani A, Albertini R, Novello E, et al. Therapeutic drug monitoring of tacrolimus-personalized therapy in heart transplantation: new strategies and preliminary results in endomyocardial biopsies. Pharmaceutics. 2022;14(6):1247.
Hurst AL, Clark N, Carpenter TC, Sundaram SS, Reiter PD. Supra-therapeutic tacrolimus concentrations associated with concomitant nicardipine in pediatric liver transplant recipients. Pediatr Transplant. 2015;19(4):E83–7.
Lee J, Kim E, Hwang S-U, Cai L, Kim M, Choi H, et al. Effect of D-glucuronic acid and N-acetyl-D-glucosamine treatment during in vitro maturation on embryonic development after parthenogenesis and somatic cell nuclear transfer in pigs. Animals. 2021;11(4):1034.
Du H, Liu P, Zhu J, Lan J, Li Y, Zhang L, et al. Hyaluronic acid-based dissolving microneedle patch loaded with methotrexate for improved treatment of psoriasis. ACS Appl Mater Interfaces. 2019;11(46):43588–98.
How KN, Yap WH, Lim CLH, Goh BH, Lai ZW. Hyaluronic acid-mediated drug delivery system targeting for inflammatory skin diseases: a mini review. Front pharmacol. 2020;11:1105.
Abdelbary AA, AbouGhaly MH. Design and optimization of topical methotrexate loaded niosomes for enhanced management of psoriasis: application of box-behnken design, in-vitro evaluation and in-vivo skin deposition study. Int J Pharm. 2015;485(1–2):235–43.
Arora D, Nanda S. Quality by design driven development of resveratrol loaded ethosomal hydrogel for improved dermatological benefits via enhanced skin permeation and retention. Int J Pharm. 2019;567: 118448.
Jain P, Taleuzzaman M, Kala C, Kumar Gupta D, Ali A, Aslam M. Quality by design (qbd) assisted development of phytosomal gel of aloe vera extract for topical delivery. J Liposome Res. 2021;31(4):381–8.
Elkomy MH, Elmowafy M, Shalaby K, Azmy AF, Ahmad N, Zafar A, et al. Development and machine-learning optimization of mucoadhesive nanostructured lipid carriers loaded with fluconazole for treatment of oral candidiasis. Drug Dev Ind Pharm. 2021;47(2):246–58.
Tkachenko Y, Niedzielski P. FTIR as a method for qualitative assessment of solid samples in geochemical research: A review. Molecules. 2022;27(24):8846.
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57.
Satyam G, Shivani S, Garima G. Ethosomes: a novel tool for drug delivery through the skin. J Pharm Res. 2010;3(4):688–91.
Pathan IB, Jaware BP, Shelke S, Ambekar W. Curcumin loaded ethosomes for transdermal application: formulation, optimization, in-vitro and in-vivo study. J Drug Deliv Sci Technol. 2018;44:49–57.
Zhang Z, Feng S-S. The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly (lactide)–tocopheryl polyethylene glycol succinate nanoparticles. Biomater. 2006;27(21):4025–33.
Inkson BJ. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) for materials characterization. In: Materials characterization using nondestructive evaluation (NDE) methods. Woodhead Publishing; 2016. p 17–43.
Hayat ME. Basic techniques for transmission electron microscopy. Academic Press 1986, The University of California. 1985;(1):67–8.
Vanaja K, Shobha Rani R, Sacchidananda S. Formulation and clinical evaluation of ultradeformable liposomes in the topical treatment of psoriasis. Clin Res Regul Aff. 2008;25(1):41–52.
Leyva-Porras C, Cruz-Alcantar P, Espinosa-Solís V, Martínez-Guerra E, Piñón-Balderrama CI, Compean Martínez I, et al. Application of differential scanning calorimetry (DSC) and modulated differential scanning calorimetry (MDSC) in food and drug industries. Polymers. 2019;12(1):5.
Durowoju IB, Bhandal KS, Hu J, Carpick B, Kirkitadze M. Differential scanning calorimetry—a method for assessing the thermal stability and conformation of protein antigen. J Vis Exp. 2017;121: e55262.
Guo T, Lu J, Fan Y, Zhang Y, Yin S, Sha X, et al. TPGS assists the percutaneous administration of curcumin and glycyrrhetinic acid coloaded functionalized ethosomes for the synergistic treatment of psoriasis. Int J Pharm. 2021;604: 120762.
Elgewelly MA, Elmasry SM, El Sayed NS, Abbas H. Resveratrol-loaded vesicular elastic nanocarriers gel in imiquimod-induced psoriasis treatment: in vitro and in vivo evaluation. J Pharm Sci. 2022;111(2):417–31.
Patel NA, Patel NJ, Patel RP. Formulation and evaluation of curcumin gel for topical application. Pharm Dev Techno. 2009;14(1):83–92.
Wohlrab J, Gebert A. pH and buffer capacity of topical formulations. pH of the skin: issues and challenges. Karger Publishers; 2018. p. 123–31.
Dantas MG, Reis SA, Damasceno CM, Rolim LA, Rolim-Neto PJ, Carvalho FO, Quintans-Junior LJ, Almeida JR. Development and evaluation of stability of a gel formulation containing the monoterpene borneol. Sci World J. 2016;2016.
Kaur M, Singh K, Jain SK. Luliconazole vesicular based gel formulations for its enhanced topical delivery. J Liposome Res. 2020;30(4):388–406.
Jain AK, Thareja S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif Cells Nanomed Biotechnol. 2019;47(1):524–39.
Bhardwaj P, Tripathi P, Pandey S, Gupta R, Khar RK, Patil PR. Improved dermal delivery of pentoxifylline niosomes for the management of psoriasis: development, optimization and in-vivo studies in imiquimod induced psoriatic plaque model. J Drug Deliv Sci Technol. 2022;75: 103643.
Khatoon K, Ali A, Ahmad FJ, Hafeez Z, Rizvi MMA, Akhter S, et al. Novel nanoemulsion gel containing triple natural bio-actives combination of curcumin, thymoquinone, and resveratrol improves psoriasis therapy: in vitro and in vivo studies. Drug Deliv Transl Res. 2021;11:1245–60.
Ahad A, Al-Saleh AA, Al-Mohizea AM, Al-Jenoobi FI, Raish M, Yassin AEB, et al. Formulation and characterization of phospholipon 90 G and tween 80 based transfersomes for transdermal delivery of eprosartan mesylate. Pharm Dev Technol. 2018;23(8):787–93.
Alam MS, Ali MS, Alam N, Siddiqui MR, Shamim M, Safhi M. In vivo study of clobetasol propionate loaded nanoemulsion for topical application in psoriasis and atopic dermatitis. Drug Invent Today. 2013;5(1):8–12.
Struck MB, Andrutis KA, Ramirez HE, Battles AH. Effect of a short-term fast on ketamine–xylazine anesthesia in rats. J Am Assoc Lab Anim Sci. 2011;50(3):344–8.
Okasha EF, Bayomy NA, Abdelaziz EZ. Effect of topical application of black seed oil on imiquimod-induced psoriasis-like lesions in the thin skin of adult male albino rats. Anat Rec. 2018;301(1):166–74.
Smajlović A, Haverić A, Alić A, Hadžić M, Smajlović A, Mujezinović I, et al. Molecular and histopathological profiling of imiquimod induced dermatosis in swiss wistar rats: contribution to the rat model for novel anti-psoriasis treatments. Mol Biol Rep. 2021;48(5):4295–303.
Chen H, Lu C, Liu H, Wang M, Zhao H, Yan Y, et al. Quercetin ameliorates imiquimod-induced psoriasis-like skin inflammation in mice via the NF-κB pathway. Int Immunopharmacol. 2017;48:110–7.
Ge Djiobie Tchienou, Rk TsatsopTsague, Tf MbamPega, Bama V, Bamseck A, DongmoSokeng S, et al. Multi-response optimization in the formulation of a topical cream from natural ingredients. Cosmetics. 2018;5(1):7.
Pawar P, Kashyap H, Malhotra S, Sindhu R. Hp--CD-voriconazole in situ gelling system for ocular drug delivery: in vitro, stability, and antifungal activities assessment. BioMed Res Int. 2013;2013.
Chawla A, Sharma P, Pawar P. Eudragit S-100 coated sodium alginate microspheres of naproxen sodium: formulation, optimization and in vitro evaluation. Acta Pharm. 2012;62(4):529–45.
Author information
Authors and Affiliations
Contributions
ND: investigation, software, validation, formal analysis, writing—original draft. A: validation, investigation, data curation. DS: methodology, resources. AS: conceptualization, methodology, resources, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Ethical Approval
The manuscript is in agreement with ethical principles.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dadwal, N., Amisha, Singh, D. et al. Quality-by-Design Approach for Investigating the Efficacy of Tacrolimus and Hyaluronic Acid–Loaded Ethosomal Gel in Dermal Management of Psoriasis: In Vitro, Ex Vivo, and In Vivo Evaluation. AAPS PharmSciTech 24, 220 (2023). https://doi.org/10.1208/s12249-023-02678-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02678-6