Abstract
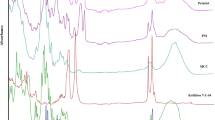
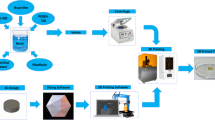
The aim of this work was to design pediatric-friendly, dose-flexible orally disintegrating drug delivery systems (printlets) of the antiviral drug tenofovir disoproxil fumarate (TDF) by selective laser sintering (SLS) for potential use in hospitals along with other antiviral drugs. In order to obtain a consistent quality of printlets with desired properties, it is important to understand certain critical quality attributes for their main and interactions effect. The printlets were optimized by Box-Behnken’s design of the experiment by varying process variables while keeping the composition constant. The composition contained 16.3% TDF, 72.7% polyvinyl pyrrolidone K16-18, 8% magnesium aluminum silicate, 3% Candurin® NXT Ruby Red, and 0.3% colloidal silicon dioxide. The process variables studied were surface (X1), chamber temperatures (X2), and laser scanning speed (X3). The range of variable levels was 75–85°C for X1, 50–70°C for X2, and 200–240 mm/s for X3, respectively. The responses studied were hardness, disintegration time, dissolution, physiochemical, and pharmacokinetic characterization. X-ray powder diffraction indicated partial or complete conversion of the crystalline drug into amorphous form in the printlets. Comparative pharmacokinetics between Viread® (generic) and printlets in rats were superimposable. Pharmacokinetic parameters showed statistically insignificant differences between the two formulations in terms of Tmax, Cmax, and AUC of (p > 0.05). Printlets were bioequivalent to Viread® as per FDA bioequivalence criteria. Thus, the SLS printing method showed the fabrication of dose-flexible printlets with quality, and in vivo performance equivalent to commercial tablets.
Graphical Abstract











Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
UNAIDS: Global HIV and AIDS statistics–fact sheet. 2021. https://www.unaids.org/en/resources/fact-sheet. Accessed 31 May 2022.
Lloyd A. HIV infection and AIDS. P N G Med J. 1996;39(3):174–80.
Kovacs L, Kress TC, de Chantemèle EJB. HIV, Combination antiretroviral therapy, and vascular diseases in men and women. JACC Basic Transl Sci. 2022;7(4):410–21. https://doi.org/10.1016/j.jacbts.2021.10.017.
Rodés B, Cadiñanos J, Esteban-Cantos A, Rodríguez-Centeno J, Arribas JR. Ageing with HIV: challenges and biomarkers. EBioMedicine. 2022;77:103896. https://doi.org/10.1016/j.ebiom.2022.103896.
Buchanan AL, Montepiedra G, Sirois PA, Kammerer B, Garvie PA, Storm DS, et al. Barriers to medication adherence in HIV-infected children and youth based on self- and caregiver report. Pediatrics. 2012;129(5):e1244–51. https://doi.org/10.1542/peds.2011-1740.
Haberer J, Mellins C. Pediatric adherence to HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2009;6(4):194–200. https://doi.org/10.1007/s11904-009-0026-8.
Schlatter AF, Deathe AR, Vreeman RC. The need for pediatric formulations to treat children with HIV. AIDS Res Treat. 2016;2016:1654938. https://doi.org/10.1155/2016/1654938.
Lee C, Sapasap J, LaRochelle J, Smith RO, Badowski ME. Antiretroviral therapy in children and adolescents: a look into modern single tablet regimens. J Pediatr Pharmacol Ther. 2021;26(8):783–94. https://doi.org/10.5863/1551-6776-26.8.783.
Lisi DM. Pros and cons of pharmacy compounding. US Pharm. 2021;46(11):8–12.
Gobetti C. Evaluation of physicochemical and microbiological stability of liquid preparation from tizanidine hydrochloride tablets-a hospital concern. Braz J Pharm Sci. 2021;57. https://doi.org/10.1590/s2175-97902020000418896.
Fina F, Madla CM, Goyanes A, Zhang J, Gaisford S, Basit AW. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int J Pharm. 2018;541(1–2):101–7. https://doi.org/10.1016/j.ijpharm.2018.02.015.
Awad A, Fina F, Trenfield SJ, Patel P, Goyanes A, Gaisford S, et al. 3D printed pellets (miniprintlets): a novel, multi-drug, controlled release platform technology. Pharmaceutics. 2019;11(4). https://doi.org/10.3390/pharmaceutics11040148.
Mohamed EM, Barakh Ali SF, Rahman Z, Dharani S, Ozkan T, Kuttolamadom MA, et al. Formulation optimization of selective laser sintering 3D-printed tablets of clindamycin palmitate hydrochloride by response surface methodology. AAPS PharmSciTech. 2020;21(6):232. https://doi.org/10.1208/s12249-020-01775-0.
Gallant JE, Deresinski S. Tenofovir disoproxil fumarate. Clin Infect Dis. 2003;37(7):944–50. https://doi.org/10.1086/378068.
Robbins BL, Srinivas RV, Kim C, Bischofberger N, Fridland A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother. 1998;42(3):612–7. https://doi.org/10.1128/aac.42.3.612.
Viread® (tenofovir disoproxil fumarate) tablets: US package insert Accessed April 22 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021356s042,022577s002lbl.pdf
Mulato AS, Cherrington JM. Anti-HIV activity of adefovir (PMEA) and PMPA in combination with antiretroviral compounds: in vitro analyses. Antiviral Res. 1997;36(2):91–7. https://doi.org/10.1016/s0166-3542(97)00043-0.
Guidelines for the use of antiretroviral agents in pediatric HIV infection. In: Services UDoHaH, editor. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/PediatricGuidelines.pdf2020.
FDA: Viread® FDA label. http://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021356s058,022577s014lbl.pdf Accessed May 31 2020.
Rahman Z, Barakh Ali SF, Ozkan T, Charoo NA, Reddy IK, Khan MA. Additive manufacturing with 3D printing: progress from bench to bedside. Aaps j. 2018;20(6):101. https://doi.org/10.1208/s12248-018-0225-6.
Barakh Ali SF, Mohamed EM, Ozkan T, Kuttolamadom MA, Khan MA, Asadi A, et al. Understanding the effects of formulation and process variables on the printlets quality manufactured by selective laser sintering 3D printing. Int J Pharm. 2019;570:118651. https://doi.org/10.1016/j.ijpharm.2019.118651.
Hamed R, Mohamed EM, Rahman Z, Khan MA. 3D-printing of lopinavir printlets by selective laser sintering and quantification of crystalline fraction by XRPD-chemometric models. Int J Pharm. 2021;592:120059. https://doi.org/10.1016/j.ijpharm.2020.120059.
Charoo NA, Barakh Ali SF, Mohamed EM, Kuttolamadom MA, Ozkan T, Khan MA, et al. Selective laser sintering 3D printing–an overview of the technology and pharmaceutical applications. Drug Dev Ind Pharm. 2020;46(6):869–77. https://doi.org/10.1080/03639045.2020.1764027.
FDA: Distributed manufacturing and point-of-care manufacturing of drugs. 2022. https://www.fda.gov/media/162157/download?attachment. Accessed 1 May 2022.
Gomes ECdL, Mussel WN, Resende JM, Fialho SL, Barbosa J, Carignani E, et al. Characterization of tenofovir disoproxil fumarate and its behavior under heating. Crystal Growth Des. 2015;15(4):1915–22. https://doi.org/10.1021/acs.cgd.5b00089.
Wu C-j, You J-z, Wang X-j. Thermal decomposition mechanism of tenofovir disoproxil fumarate. J Therm Anal Calorim. 2018;132(1):471–82. https://doi.org/10.1007/s10973-017-6910-3.
Rahman Z, Zidan AS, Habib MJ, Khan MA. Understanding the quality of protein loaded PLGA nanoparticles variability by Plackett-Burman design. Int J Pharm. 2010;389(1–2):186–94. https://doi.org/10.1016/j.ijpharm.2009.12.040.
Alyami H, Koner J, Huynh C, Terry D, Mohammed AR. Current opinions and recommendations of paediatric healthcare professionals–the importance of tablets: emerging orally disintegrating versus traditional tablets. PLOS ONE. 2018;13(2):e0193292. https://doi.org/10.1371/journal.pone.0193292.
Charoo NA, Rahman Z, Ali AA. Is the demonstration of bioequivalence for clavulanic acid required in amoxicillin-clavulanic acid orally administered immediate-release products? J Pharm Pharmacol. 2018;70(7):883–92. https://doi.org/10.1111/jphp.12920.
FDA: Guidance for industry–orally disintegrating tablets. 2008. https://www.fda.gov/media/70877/download. Accessed 31 May 2022.
FDA: Dissolution methods–tenofovir disoroxil fumerate. https://www.accessdata.fda.gov/scripts/cder/dissolution/dsp_SearchResults.cfm. Accessed 6 June 2022.
Lee EH, Smith DT, Fanwick PE, Byrn SR. Characterization and anisotropic lattice expansion/contraction of polymorphs of tenofovir disoproxil fumarate. Cryst Growth Des. 2010;10(5):2314–22. https://doi.org/10.1021/cg1000667.
Daimay Lin-Vien NC, William Fateley, Jeanette Grasselli. The handbook of infrared and raman characteristic frequencies of organic molecules. 1st ed. Academic Press; 1991.
Saroj AL, Singh RK, Chandra S. Studies on polymer electrolyte poly(vinyl) pyrrolidone (PVP) complexed with ionic liquid: effect of complexation on thermal stability, conductivity and relaxation behaviour. Mater Sci Eng, B. 2013;178(4):231–8. https://doi.org/10.1016/j.mseb.2012.11.007.
Sharma A, Jain CP. Preparation and characterization of solid dispersions of carvedilol with PVP K30. Res Pharm Sci. 2010;5(1):49–56.
Dharani S, Barakh Ali SF, Afrooz H, Mohamed EM, Cook P, Khan MA, et al. Development of methamphetamine abuse-deterrent formulations using sucrose acetate isobutyrate. J Pharm Sci. 2020;109(3):1338–46. https://doi.org/10.1016/j.xphs.2019.12.003.
Lee J, Boerrigter SX, Jung YW, Byun Y, Yuk SH, Byrn SR, et al. Organic vapor sorption method of isostructural solvates and polymorph of tenofovir disoproxil fumarate. Eur J Pharm Sci. 2013;50(3–4):253–62. https://doi.org/10.1016/j.ejps.2013.07.004.
Buera MDP, Levi G, Karel M. Glass transition in poly(vinylpyrrolidone): effect of molecular weight and diluents. Biotechnol Progress. 1992;8(2):144–8. https://doi.org/10.1021/bp00014a008.
Slade L, Levine H. Beyond water activity: recent advances based on an alternative approach to the assessment of food quality and safety. Crit Rev Food Sci Nutr. 1991;30(2–3):115–360.
Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet. 2004;43(9):595–612. https://doi.org/10.2165/00003088-200443090-00003.
Podany AT, Bares SH, Havens J, Dyavar SR, O’Neill J, Lee S, et al. Plasma and intracellular pharmacokinetics of tenofovir in patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. AIDS. 2018;32(6):761–5. https://doi.org/10.1097/qad.0000000000001744.
Tourret J, Deray G, Isnard-Bagnis C. Tenofovir effect on the kidneys of HIV-infected patients: a double-edged sword? J Am Soc Nephrol. 2013;24(10):1519–27. https://doi.org/10.1681/asn.2012080857.
FDA: guidance document- bioequivalence studies with pharmacokinetic endpoints for drugs submitted under an ANDA guidance for industry. 2021. https://www.fda.gov/media/87219/download. Accessed May 31, 2022.
Acknowledgements
The use of the TAMU materials characterization facility is acknowledged for allowing us to use SEM.
Funding
This work was supported by National Institute of Health R56 grant #1R56HD106612, and partly by R01 grant #1R01HD112077.
Author information
Authors and Affiliations
Contributions
Canberk Kayalar: conceptualization, validation, investigation, visualization, writing—review and editing
Nada Helal: validation, investigation, visualization
Sathish Dharani: validation, investigation, visualization
Eman M. Mohamed: validation, investigation, visualization
Tahir Khuroo: validation, investigation, visualization
Mathew A. Kuttolamadom: conceptualization, formal analysis, writing—review and editing
Ziyaur Rahman: conceptualization, formal analysis, writing—review and editing, supervision, project administration
Mansoor A. Khan: conceptualization, formal analysis, writing—review and editing, supervision, project administration
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kayalar, C., Rahman, Z., Mohamed, E.M. et al. Preparation and Characterization of 3D-Printed Dose-Flexible Printlets of Tenofovir Disoproxil Fumarate. AAPS PharmSciTech 24, 171 (2023). https://doi.org/10.1208/s12249-023-02623-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02623-7