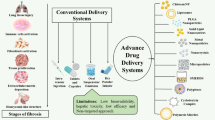
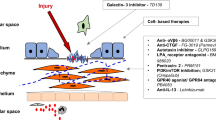
Abstract
Idiopathic pulmonary fibrosis (IPF) is an ailment with no cure and a very high rate of progression that ultimately leads to death. The exact reason for this disease is still not acknowledged. Many underlying mechanisms of wound healing and various types of stimuli that trigger the pathogenesis of IPF continue to be intensively explored. The exact therapy for the reversal of this disease is not yet known and is constantly in progress. Existing treatments only slow down the process or mitigate the symptoms to enhance the patient’s healthcare system. The only two Food and Drug Administration-approved oral medications include pirfenidone and nintedanib whose high dose and systemic circulation can have side effects to a greater extent. Further research on restorative and extra-curative therapies for IPF is necessary due to the absence of viable therapeutic choices. To assure minimum off-targeted site delivery and longer duration of action, techniques that offer a sustainable release of the drug, better bioavailability, and patient compliance can be used.
The work is an overview of the main therapeutic targets and pertinent developing therapies for the management of IPF. This study is an attempt to focus on various drug delivery systems that are responsible for showing effectiveness in defense mechanisms against IPF.
Graphical abstract


Similar content being viewed by others
References
Ibarra-Sánchez LÁ, Gámez-Méndez A, Martínez-Ruiz M, Nájera-Martínez EF, Morales-Flores BA, Melchor-Martínez EM, et al. Nanostructures for drug delivery in respiratory diseases therapeutics: revision of current trends and its comparative analysis. J Drug Deliv Sci Technol. 2022;70:103219. https://doi.org/10.1016/j.jddst.2022.103219.
Barratt SL, Creamer A, Hayton C, Chaudhuri N. Idiopathic pulmonary fibrosis (IPF): an overview. J Clin Med. 2018;7:201. https://doi.org/10.3390/jcm7080201.
Raghu G, Mehta S. Interstitial lung disease (ILD) in India: insights and lessons from the prospective, landmark ILD-India registry. Lung India. 2016;33:589-591. https://doi.org/10.4103/0970-2113.192874.
Higashiyama H, Yoshimoto D, Kaise T, Matsubara S, Fujiwara M, Kikkawa H, et. al. Inhibition of activin receptor-like kinase 5 attenuates bleomycin-induced pulmonary fibrosis. Exp. Mol. Pathol. 2007;83:39-46. https://doi.org/10.1016/j.yexmp.2006.12.003.
Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. https://doi.org/10.1038/nrdp.2017.74.
George PM, Patterson CM, Reed AK, Thillai M. Lung transplantation for idiopathic pulmonary fibrosis. Lancet Respir Med. 2019;7:271-282. https://doi.org/10.1016/S2213-2600(18)30502-2.
Dudala SS, Venkateswarulu TC, Kancharla SC, Kodali VP, Babu DJ. A review on importance of bioactive compounds of medicinal plants in treating idiopathic pulmonary fibrosis (special emphasis on isoquinoline alkaloids). Future J. Pharm. Sci. 2021;7:1-20. https://doi.org/10.1186/s43094-021-00304-5
Selman M, King TE, Pardo A. American Thoracic Society; European Respiratory Society; American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136-51. https://doi.org/10.7326/0003-4819-134-2-200101160-00015.
Phan THG, Paliogiannis P, Nasrallah GK, Giordo R, Eid AH, Fois AG, et al. Emerging cellular and molecular determinants of idiopathic pulmonary fibrosis. Cell Mol Life Sci. 2021 Mar;78:2031-2057. https://doi.org/10.1007/s00018-020-03693-7.
Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528-42. https://doi.org/10.1177/147323000903700531.
Gosain A, DiPietro LA. Aging and wound healing. World J. Surg. 2004;28:321-6. https://doi.org/10.1007/s00268-003-7397-6
Mofazzal Jahromi MA, Sahandi Zangabad P, Moosavi Basri SM, Sahandi Zangabad K, Ghamarypour A, Aref AR, et al. Nanomedicine and advanced technologies for burns: preventing infection and facilitating wound healing. Adv Drug Deliv Rev. 2018;123:33-64. https://doi.org/10.1016/j.addr.2017.08.001.
Campos AC, Groth AK, Branco AB. Assessment and nutritional aspects of wound healing. Curr Opin Clin Nutr Metab Care. 2008;11:281-8. https://doi.org/10.1097/MCO.0b013e3282fbd35a.
Hinz B, Lagares D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol. 2020;16:11-31. https://doi.org/10.1038/s41584-019-0324-5.
Bochaton-Piallat ML, Gabbiani G, Hinz B. The myofibroblast in wound healing and fibrosis: answered and unanswered questions. F1000Res. 2016;5. https://doi.org/10.12688/f1000research.8190.1.
Henry G, Garner WL. Inflammatory mediators in wound healing. Surg Clin North Am. 2003;83:483-507. https://doi.org/10.1016/S0039-6109(02)00200-1.
Walton KL, Johnson KE, Harrison CA. Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Front Pharmacol. 2017;8:461. https://doi.org/10.3389/fphar.2017.00461.
Piersma B, Bank RA, Boersema M. Signaling in fibrosis: TGF-β, WNT, and YAP/TAZ converge. Front Med (Lausanne). 2015;2:59. https://doi.org/10.3389/fmed.2015.00059.
Shen X, Peng Y, Li H. The injury-related activation of hedgehog signaling pathway modulates the repair-associated inflammation in liver fibrosis. Front Immunol. 2017;8:1450. https://doi.org/10.3389/fimmu.2017.01450.
Hu B, Phan SH. Notch in fibrosis and as a target of anti-fibrotic therapy. Pharmacol Res. 2016;108:57-64. https://doi.org/10.1016/j.phrs.2016.04.010.
Martinon F. Detection of immune danger signals by NALP3. J Leukoc Biol. 2008;83:507-11. https://doi.org/10.1189/jlb.0607362.
Sontake V, Gajjala PR, Kasam RK, Madala SK. New therapeutics based on emerging concepts in pulmonary fibrosis. Expert Opin Ther Targets. 2019;23:69–81. https://doi.org/10.1080/14728222.2019.1552262.
Zhang J, Wang T, Saigal A, Johnson J, Morrisson J, Tabrizifard S, et al. Discovery of a new class of integrin antibodies for fibrosis. Sci Rep. 2021;11:2118. https://doi.org/10.1038/s41598-021-81253-0.
John AE, Graves RH, Pun KT, Vitulli G, Forty EJ, Mercer PF, et al. Translational pharmacology of an inhaled small molecule αvβ6 integrin inhibitor for idiopathic pulmonary fibrosis. Nat Commun. 2020;11:4659. https://doi.org/10.1038/s41467-020-18397-6.
Conte E, Fruciano M, Fagone E, Gili E, Caraci F, Iemmolo M, et al. Inhibition of PI3K prevents the proliferation and differentiation of human lung fibroblasts into myofibroblasts: the role of class I P110 isoforms. PLoS One. 2011;6:e24663. https://doi.org/10.1371/journal.pone.0024663.
Zhang XL, Xing RG, Chen L, Liu CR, Miao ZG. PI3K/Akt signaling is involved in the pathogenesis of bleomycin-induced pulmonary fibrosis via regulation of epithelial-mesenchymal transition. Mol Med Rep. 2016;14:5699-5706. https://doi.org/10.3892/mmr.2016.5960.
Milara J, Ballester B, Morell A, Ortiz JL, Escrivá J, Fernández E, et al. JAK2 mediates lung fibrosis, pulmonary vascular remodelling and hypertension in idiopathic pulmonary fibrosis: an experimental study. Thorax. 2018;73:519-529. https://doi.org/10.1136/thoraxjnl-2017-210728.
Mullally A, Hood J, Harrison C, Mesa R. Fedratinib in myelofibrosis. Blood Adv. 2020;4:1792-1800. https://doi.org/10.1182/bloodadvances.2020002897.
d'Alessandro M, Perillo F, Metella Refini R, Bergantini L, Bellisai F, Selvi E, et al. Efficacy of baricitinib in treating rheumatoid arthritis: modulatory effects on fibrotic and inflammatory biomarkers in a real-life setting. Int Immunopharmacol. 2020;86:106748. https://doi.org/10.1016/j.intimp.2020.106748.
Ruan H, Luan J, Gao S, Li S, Jiang Q, Liu R, et al. Fedratinib attenuates bleomycin-induced pulmonary fibrosis via the JAK2/STAT3 and TGF-β1 signaling pathway. Molecules. 2021;26:4491. https://doi.org/10.3390/molecules26154491.
Epstein Shochet G, Brook E, Bardenstein-Wald B, Shitrit D. TGF-β pathway activation by idiopathic pulmonary fibrosis (IPF) fibroblast derived soluble factors is mediated by IL-6 trans-signaling. Respir Res. 2020;21:56. https://doi.org/10.1186/s12931-020-1319-0.
Zhang Y, Liang R, Chen CW, Mallano T, Dees C, Distler A, et al. JAK1-dependent transphosphorylation of JAK2 limits the antifibrotic effects of selective JAK2 inhibitors on long-term treatment. Ann Rheum Dis. 2017;76:1467-1475. https://doi.org/10.1136/annrheumdis-2016-210911.
Fujimoto H, Kobayashi T, Azuma A. Idiopathic pulmonary fibrosis: treatment and prognosis. Clin Med Insights Circ Respir Pulm Med. 2016;9:179-185. https://doi.org/10.4137/CCRPM.S23321.
Spagnolo P, Del Giovane C, Luppi F, Cerri S, Balduzzi S, Walters EH, et al. Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2010;CD003134. https://doi.org/10.1002/14651858.CD003134.pub2.
Strieter RM, Mehrad B. New mechanisms of pulmonary fibrosis. Chest. 2009;136:1364-1370. https://doi.org/10.1378/chest.09-0510
Jo HE, Randhawa S, Corte TJ, Moodley Y. Idiopathic pulmonary fibrosis and the elderly: diagnosis and management considerations. Drugs Aging. 2016 May;33(5):321–34. https://doi.org/10.1007/s40266-016-0366-1.
Ghumman M, Dhamecha D, Gonsalves A, Fortier L, Sorkhdini P, Zhou Y, et al. Emerging drug delivery strategies for idiopathic pulmonary fibrosis treatment. Eur J Pharm Biopharm. 2021;164:1-12. https://doi.org/10.1016/j.ejpb.2021.03.017.
Hawthorne D, Pannala A, Sandeman S, Lloyd A. Sustained and targeted delivery of hydrophilic drug compounds: a review of existing and novel technologies from bench to bedside. J Drug Deliv Sci Technol. 2022:103936. https://doi.org/10.1016/j.jddst.2022.103936.
Bahadur S, Jha MK. Emerging nanoformulations for drug targeting to brain through intranasal delivery: a comprehensive review. J Drug Deliv Sci Technol. 2022:103932. https://doi.org/10.1016/j.jddst.2022.103932
Rasooli R, Rajaian H, Pardakhty A, Mandegary A. Preference of aerosolized pirfenidone to oral intake: an experimental model of pulmonary fibrosis by paraquat. J Aerosol Med Pulm Drug Deliv. 2018;31:25-32. https://doi.org/10.1089/jamp.2016.1342.
Zielińska A, Carreiró F, Oliveira AM, Neves A, Pires B, Venkatesh DN, et al. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25:3731. https://doi.org/10.3390/molecules25163731.
Banik BL, Fattahi P, Brown JL. Polymeric nanoparticles: the future of nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:271-99. https://doi.org/10.1002/wnan.1364.
Keum H, Kim D, Kim J, Kim TW, Whang CH, Jung W, et al. A bilirubin-derived nanomedicine attenuates the pathological cascade of pulmonary fibrosis. Biomaterials. 2021;275:120986. https://doi.org/10.1016/j.biomaterials.2021.120986.
Saghir SAM, Al-Gabri NA, Khafaga AF, El-Shaer NH, Alhumaidh KA, Elsadek MF, et al. Thymoquinone-PLGA-PVA nanoparticles ameliorate bleomycin-induced pulmonary fibrosis in rats via regulation of inflammatory cytokines and iNOS signaling. Animals (Basel). 2019;9:951. https://doi.org/10.3390/ani9110951.
Abnoos M, Mohseni M, Mousavi SAJ, Ashtari K, Ilka R, Mehravi B. Chitosan-alginate nano-carrier for transdermal delivery of pirfenidone in idiopathic pulmonary fibrosis. Int J Biol Macromol. 2018;118:1319-1325. https://doi.org/10.1016/j.ijbiomac.2018.04.147.
Tang J, Li J, Li G, Zhang H, Wang L, Li D, et al. Spermidine-mediated poly(lactic-co-glycolic acid) nanoparticles containing fluorofenidone for the treatment of idiopathic pulmonary fibrosis. Int J Nanomedicine. 2017;12:6687-6704. https://doi.org/10.2147/IJN.S140569.
Liu L, Ren J, He Z, Men K, Mao Y, Ye T, et al. Cholesterol-modified hydroxychloroquine-loaded nanocarriers in bleomycin-induced pulmonary fibrosis. Sci Rep. 2017;7:1-1. https://doi.org/10.1038/s41598-017-11450-3
Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5:123-127.10.1007/s13205-014-0214-0
Galdino de Souza D, Santos DS, Simon KS, Morais JAV, Coelho LC, Pacheco TJA, et al. Fish oil nanoemulsion supplementation attenuates bleomycin-induced pulmonary fibrosis BALB/c mice. Nanomaterials (Basel). 2022;12:1683. https://doi.org/10.3390/nano12101683.
Ding L, Tang S, Tang W, Mosley DD, Yu A, Sil D, et al. Perfluorocarbon nanoemulsions enhance therapeutic siRNA delivery in the treatment of pulmonary fibrosis. Adv Sci (Weinh). 2022;9:e2103676. https://doi.org/10.1002/advs.202103676.
Zhang G, Xie F, Sun Y, Yu X, Xiao Z, Fang R, et al. Inhalable jojoba oil dry nanoemulsion powders for the treatment of lipopolysaccharide- or H2O2-induced acute lung injury. Pharmaceutics. 2021;13:486. https://doi.org/10.3390/pharmaceutics13040486.
Chung EP, Wells AR, Kiamco MM, Leung KP. Dual asymmetric centrifugation efficiently produces a poloxamer-based nanoemulsion gel for topical delivery of pirfenidone. AAPS PharmSciTech. 2020;21:265. https://doi.org/10.1208/s12249-020-01798-7.
Panahi Y, Farshbaf M, Mohammadhosseini M, Mirahadi M, Khalilov R, Saghfi S, et al. Recent advances on liposomal nanoparticles: synthesis, characterization and biomedical applications. Artif Cells Nanomed Biotechnol. 2017;45:788-799. https://doi.org/10.1080/21691401.2017.1282496.
Peralta MF, Guzmán ML, Pérez AP, Apezteguia GA, Fórmica ML, Romero EL, et al. Liposomes can both enhance or reduce drugs penetration through the skin. Sci Rep. 2018;8:1-1. https://doi.org/10.1038/s41598-018-31693-y
Han M, Song Y, Liu S, Lu X, Su L, Liu M, et al. Engineering of stimulus-responsive pirfenidone liposomes for pulmonary delivery during treatment of idiopathic pulmonary fibrosis. Front Pharmacol. 2022;13:882678. https://doi.org/10.3389/fphar.2022.882678.
Kotta S, Aldawsari HM, Badr-Eldin SM, Binmahfouz LS, Bakhaidar RB, Sreeharsha N, et al. Aerosol delivery of surfactant liposomes for management of pulmonary fibrosis: an approach supporting pulmonary mechanics. Pharmaceutics. 2021;13:1851. https://doi.org/10.3390/pharmaceutics13111851.
Sang X, Wang Y, Xue Z, Qi D, Fan G, Tian F, et al. Macrophage-targeted lung delivery of dexamethasone improves pulmonary fibrosis therapy via regulating the immune microenvironment. Front Immunol. 2021;12:613907. https://doi.org/10.3389/fimmu.2021.613907.
Pan T, Zhou Q, Miao K, Zhang L, Wu G, Yu J, et al. Suppressing Sart1 to modulate macrophage polarization by siRNA-loaded liposomes: a promising therapeutic strategy for pulmonary fibrosis. Theranostics. 2021;11:1192-1206. https://doi.org/10.7150/thno.48152.
Elkomy MH, Khallaf RA, Mahmoud MO, Hussein RRS, El-Kalaawy AM, Abdel-Razik AH, et al. Intratracheally inhalable nifedipine-loaded chitosan-plga nanocomposites as a promising nanoplatform for lung targeting: snowballed protection via regulation of TGF-β/β-catenin pathway in bleomycin-induced pulmonary fibrosis. Pharmaceuticals (Basel). 2021;14:1225. https://doi.org/10.3390/ph14121225.
Wang Z, Cuddigan JL, Gupta SK, Meenach SA. Nanocomposite microparticles (nCmP) for the delivery of tacrolimus in the treatment of pulmonary arterial hypertension. Int J Pharm. 2016;512:305-313. https://doi.org/10.1016/j.ijpharm.2016.08.047.
Wang Z, Meenach SA. Synthesis and characterization of nanocomposite microparticles (nCmP) for the treatment of cystic fibrosis-related infections. Pharmaceutical research. 2016;33:1862-72. https://doi.org/10.1007/s11095-016-1921-5.
Stocke NA, Meenach SA, Arnold SM, Mansour HM, Hilt JZ. Formulation and characterization of inhalable magnetic nanocomposite microparticles (MnMs) for targeted pulmonary delivery via spray drying. Int J Pharm. 2015;479:320-8. https://doi.org/10.1016/j.ijpharm.2014.12.050.
Telko MJ, Hickey AJ. Dry powder inhaler formulation. Respir Care. 2005;50:1209-27.
Lu P, Li J, Liu C, Yang J, Peng H, Xue Z, et al. Salvianolic acid B dry powder inhaler for the treatment of idiopathic pulmonary fibrosis. Asian J Pharm Sci. 2022;17:447-461. https://doi.org/10.1016/j.ajps.2022.04.004.
Muralidharan P, Jones B, Allaway G, Biswal SS, Mansour HM. Design and development of innovative microparticulate/nanoparticulate inhalable dry powders of a novel synthetic trifluorinated chalcone derivative and Nrf2 agonist. Sci Rep. 2020;10:19771. https://doi.org/10.1038/s41598-020-76585-2.
Chennakesavulu S, Mishra A, Sudheer A, Sowmya C, Suryaprakash Reddy C, Bhargav E. Pulmonary delivery of liposomal dry powder inhaler formulation for effective treatment of idiopathic pulmonary fibrosis. Asian J Pharm Sci. 2018;13:91-100. https://doi.org/10.1016/j.ajps.2017.08.005.
Trotta V, Lee WH, Loo CY, Young PM, Traini D, Scalia S. Co-spray dried resveratrol and budesonide inhalation formulation for reducing inflammation and oxidative stress in rat alveolar macrophages. Eur J Pharm Sci. 2016;86:20-8. https://doi.org/10.1016/j.ejps.2016.02.018.
Ahmed EM, Aggor FS, Awad AM, El-Aref AT. An innovative method for preparation of nanometal hydroxide superabsorbent hydrogel. Carbohydr Polym. 2013;91:693-8. https://doi.org/10.1016/j.carbpol.2012.08.056.
Shamskhou EA, Kratochvil MJ, Orcholski ME, Nagy N, Kaber G, Steen E, et al. Hydrogel-based delivery of Il-10 improves treatment of bleomycin-induced lung fibrosis in mice. Biomaterials. 2019;203:52-62. https://doi.org/10.1016/j.biomaterials.2019.02.017.
Arai T, Inoue Y, Sasaki Y, Tachibana K, Nakao K, Sugimoto C, et al. Predictors of the clinical effects of pirfenidone on idiopathic pulmonary fibrosis. Respir Investig. 2014;52:136-43. https://doi.org/10.1016/j.resinv.2013.09.002.
Jose A, Mandapalli PK, Venuganti VV. Liposomal hydrogel formulation for transdermal delivery of pirfenidone. J Liposome Res. 2016;26:139-47. https://doi.org/10.3109/08982104.2015.1060611.
Du J, El-Sherbiny IM, Smyth HD. Swellable ciprofloxacin-loaded nano-in-micro hydrogel particles for local lung drug delivery. AAPS PharmSciTech. 2014;15:1535-44. https://doi.org/10.1208/s12249-014-0176-x.
Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin FH, et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomaterials research. 2019;23:1-29. https://doi.org/10.1186/s40824-019-0166-x
van Rijt SH, Bein T, Meiners S. Medical nanoparticles for next-generation drug delivery to the lungs. Eur Respir J. 2014;44:765-74. https://doi.org/10.1183/09031936.00212813.
Acknowledgements
The authors are grateful to the Amity Institute of Pharmacy at Amity University, Noida for providing the resources and support in completion of this paper.
Author information
Authors and Affiliations
Contributions
Kirti Aggarwal: data curation, writing—original draft preparation, reviewing, and editing
Dr. Sandeep Arora: topic proposal and reviewing
Dr. Kalpana Nagpal: conceptual data suggestion reviewing, editing, writing—final editing, and revision
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aggarwal, K., Arora, S. & Nagpal, K. Pulmonary Fibrosis: Unveiling the Pathogenesis, Exploring Therapeutic Targets, and Advancements in Drug Delivery Strategies. AAPS PharmSciTech 24, 152 (2023). https://doi.org/10.1208/s12249-023-02618-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02618-4