Abstract
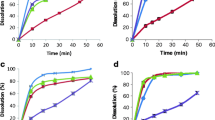
The development of formulations adapted to the patient’s age is a challenge in the pharmaceutical industry. Pediatric and geriatric patients may have difficulties in swallowing oral medications when an adequate formulation is not available. Carvedilol is a poorly water-soluble drug used to treat cardiovascular problems; it is commercialized in several countries only as solid oral formulations, which are often manipulated at the point of administration to treat pediatric or geriatric patients. The purpose of this work was to obtain a new dosage form of Carvedilol using safe excipients, suitable for administration to pediatric and geriatric patients. To improve the solubility of Carvedilol, the effect of several factors was analyzed and optimized. Subsequently, to improve the physical stability of the formulations, two preparation methods were analyzed by adding HPMC. In “method 1,” HPMC was dissolved in buffer and incorporated into a mixture of Carvedilol-PEG 400, while in “method 2,” Carvedilol was solubilized in buffer containing PEG 400, and then, HPMC was added. Finally, microbiological tests were performed to the stable formulations. The factors “pH value” and “concentration of PEG” affected the solubility of Carvedilol. A formulation containing Carvedilol (3 mg/mL), pH=3, PEG 400 (15% v/v), and HPMC (0.25% w/v) prepared by method 2 was stable for 180 days at 4 °C while those containing Carvedilol (5 mg/mL), pH=3, PEG 400 (27% v/v), and HPMC (0.5% w/v), prepared by method 2, were stable for 180 days at 4 and 25°C. These oral liquid formulations were physicochemical and microbiologically stable for 6 months.
Graphical abstract



Similar content being viewed by others
References
Ivanovska V, Rademaker CMA, van Dijk L, Mantel-Teeuwisse AK. Pediatric drug formulations: a review of challenges and progress. Pediatrics. 2014;134:361–72. https://doi.org/10.1542/peds.2013-32251.
Mieiro L, Beuscart J-B, Knol W, Van Riet-Nales D, Orlu M. Achieving appropriate medication for older adults: a multidimensional perspective. Maturitas. 2019;124:43–7. https://doi.org/10.1016/j.maturitas.2019.03.0071.
Walshe M. Oropharyngeal dysphagia in neurodegenerative disease. J Gastroenterol Hepatol Res. 2014;3:1265–71. https://doi.org/10.6051/j.issn.2224-3992.2014.03.408-21.
Hanning SM, Lopez FL, Wong ICK, Ernest TB, Tuleu COGM. Patient centric formulations for paediatrics and geriatrics: similarities and differences. Int J Pharm. 2016;9.1:512–355.
Lopez FL, Ernest TB, Tuleu C, Gul MO. Formulation approaches to pediatric oral drug delivery: benefits and limitations of current platforms. Exp Opin Drug Deliv. 2015;12:1727–40. https://doi.org/10.1517/17425247.2015.10602181.
Galanopoulou O, Rozou S, Antoniadou-Vyza E. HPLC analysis, isolation and identification of a new degradation product in carvedilol tablets. J Pharma Biomed Anal. 2008;48:70–7. https://doi.org/10.1016/j.jpba.2008.05.0041.
Loftsson T, Vogensen SB, Desbos C, Jansook P. Carvedilol: solubilization and cyclodextrin complexation: a technical note. AAPS PharmSciTech. 2008;9:425–30. https://doi.org/10.1208/s12249-008-9055-71.
Masarone D, Valente F, Rubino M, et al. Pediatric heart failure: a practical guide to diagnosis and management. Pediatr Neonatol. 2017;58:303–12. https://doi.org/10.1016/j.pedneo.2017.01.0011.
FDA Listing of Authorized Generics 2022. Available at https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/fda-listing-authorized-generics1.
Carvedilol. Available at https://medlineplus.gov/spanish/druginfo/meds/a697042-es.html1.
Menon TV, Sajeeth CL. Formulation and evaluation of sustained release sodium alginate microbeads of Carvedilol. J Pharm Technol. 2013;6:392–397.1.
Krstić M, Radojević M, Stojanović D, Radojević V, Uskoković P, Ibrić S. Formulation and characterization of nanofibers and films with carvedilol prepared by electrospinning and solution casting method. Eur J Pharm Sci. 2017;101:160–6. https://doi.org/10.1016/j.ejps.2017.02.0061.
Han H, Li Y, Peng Z, et al. A Soluplus/Poloxamer 407-based self-nanoemulsifying drug delivery system for the weakly basic drug carvedilol to improve its bioavailability. Nanomed Nanotechnol Biol Med. 2020;27:102199. https://doi.org/10.1016/j.nano.2020.1021991.
Mishra A, Imam SS, Aqil M, et al. Carvedilol nano lipid carriers: formulation, characterization and in-vivo evaluation. Drug Deliv. 2016;23:1486–94. https://doi.org/10.3109/10717544.2016.11653141.
Wegmann M, Parola L, Bertera FM, et al. Novel carvedilol paediatric nanomicelle formulation: in-vitro characterization and in-vivo evaluation. J Pharm Pharmacol. 2017;69:544–53. https://doi.org/10.1111/jphp.126051.
Medarević D, Djuriš J, Ibrić S, Mitrić M, Kachrimanis K. Optimization of formulation and process parameters for the production of carvedilol nanosuspension by wet media milling. Int J Pharm. 2018;540:150–61. https://doi.org/10.1016/j.ijpharm.2018.02.0111.
Buontempo F, Bernabeu E, Glisoni RJ, Quiroga E, DA BCC. Carvedilol stability in paediatric oral liquid formulations. Farmacia Hospitalaria. 2010;34:293–7.1.
(ICH) INTERNATIONAL CONFERENCE ON HARMONISATION. Validation of analytical procedures Q2(R2), in: 2022.1. https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline
Patel RP, Wanandy T, Zani R, Jose MD, Shastri M, Zaidi STR. Stability of trimethoprim in newly formulated liquid dosage form. J Pharm Pract Res. 2016;46:10–4. https://doi.org/10.1002/jppr.11271.
Ellis DP, Rozek T, Milne RW. Stability of a compounded oral liquid formulation of clopidogrel for infants. J Pharm Pract Res. 2020;50:321–8. https://doi.org/10.1002/jppr.16121.
European Pharmacopoeia. European Directorate for the Quality of Medicines EDQM, seventh ed. Strasbourg; 2010. pp. 163–167, 519–520.
Morri M, Casabonne C, Leonardi D., Vignaduzzo S. Orphan formulations for pediatric use: development and stability control of two sildenafil citrate solutions for the treatment of pulmonary hypertension. AAPS Pharmscitech. 2020:21. https://doi.org/10.1208/s12249-020-01768-z1.
Hamed R, Awadallah A, Sunoqrot S, et al. pH-dependent solubility and dissolution behavior of carvedilol—case example of a weakly basic BCS class II drug. AAPS PharmSciTech. 2016;17:418–26. https://doi.org/10.1208/s12249-015-0365-21.
Santos Souza HF, Real D, Leonardi D, et al. Development and in vitro/in vivo evaluation of a novel benznidazole liquid dosage form using a quality-by-design approach. TM & IH. 2017;22:1514–22. https://doi.org/10.1111/tmi.129801.
Provenza N, Calpena AC, Mallandrich M, Halbaut L, Clares B. Design and physicochemical stability studies of paediatric oral formulations of sildenafil. Int J Pharm. 2014;460:234–9. https://doi.org/10.1016/j.ijpharm.2013.11.0061.
Morri M, Castellano P, Leonardi D, Vignaduzzo S. First development, optimization, and stability control of a pediatric oral atenolol formulation. AAPS PharmSciTech. 2018;19:1781–8. https://doi.org/10.1208/s12249-018-0992-51.
Plummer DT. Introducción a la bioquímica práctica (No. 574.192 P5Y 1981). 1981. https://books.google.com.co/books?id=LFzyWzsCVlsC&printsec=frontcover#v=onepage&q&f=false
van der Vossen AC, van der Velde I, Smeets OSNM, et al. Formulating a poorly water soluble drug into an oral solution suitable for paediatric patients; lorazepam as a model drug. Eur J Pharm Sci. 2017;100:205–10. https://doi.org/10.1016/j.ejps.2017.01.0251.
Strickley RG. Solubilizing excipients used in commercially available oral and injectable formulations. Pharm Res. 2004;21:201–230.1.
(ICH) INTERNATIONAL CONFERENCE ON HARMONISATION, Q1A (R2) Stability testing of new drug substances and drug products In:2003. Available at https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-drug-products-scientific-guideline%0A1.
Raghavan S, Trividic A, Davis A, Hadgraft J. Crystallization of hydrocortisone acetate: influence of polymers. Int J Pharm. 2001;212:213–21. https://doi.org/10.1016/S0378-5173(00)00610-41.
(ICH) INTERNATIONAL CONFERENCE ON HARMONISATION. Evaluation for Stability Data Q1E, 2003. https://database.ich.org/sites/default/files/Q1EGuideline.pdf.
European Medicines Agency. Reflection paper: formulation of choice for the paediatric population (EMEA/CHMP/PEG/194810/2005). Eur Med Agency. EMEA/CHMP/:1–45.1. 2006. https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-formulations-choice-paediatricpopulation_en.pdf
Ortega O, Martín A, Clavé P. Diagnosis and management of oropharyngeal dysphagia among older persons, state of the art. J Am Med Dir Assoc. 2017;18:576–82. https://doi.org/10.1016/j.jamda.2017.02.0151.
Siddiqi N, Shatat IF. Antihypertensive agents: a long way to safe drug prescribing in children. Pediatric Nephrol. 2020;35:2049–65. https://doi.org/10.1007/s00467-019-04314-71.
Leonetti Luparini R, Celli V, Piccirillo G, Guidi V, Cacciafesta M, Marigliano V. Carvedilol in elderly patients with chronic heart failure, a 12 weeks randomized, placebo controlled open trial. Arch Gerontol Geriatr. 2000;29:275–82. https://doi.org/10.1016/S0167-4943(99)00040-01.
Funding
The authors gratefully acknowledge the Universidad Nacional de Rosario Argentina and CONICET (PUE 2016), Ministerio de Producción, Ciencia y Tecnología de Santa Fe (ASACTEI) Argentina for financial support.
Author information
Authors and Affiliations
Contributions
M. A. Operto: conception and design of study, analysis and interpretation of data, and acquisition of data, R.M. Maggio.: analysis and interpretation of data, S. E. Vignaduzzo interpretation of data, and drafting the manuscript, D. Leonardi: writing and revising the manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors report no declarations of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Operto, M.A., Maggio, R., Leonardi, D. et al. Flexible New Dosage Forms Containing Carvedilol for the Treatment of Patients with Cardiovascular Disorders: Development, Stability, Palatability, and Microbiological Studies. AAPS PharmSciTech 24, 159 (2023). https://doi.org/10.1208/s12249-023-02612-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02612-w