Abstract
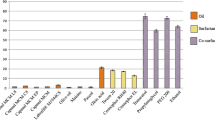
The aim of the study was to develop and optimise drug-in-adhesive (DIA) transdermal patch of duloxetine HCl for enhanced drug delivery. DIA patch so developed reduced the dose and dosing frequency by enhancing bio-performance of the drug. A transdermal DIA patch having Duro-Tak 87–2287 as DIA polymer and Transcutol P as permeation enhancer loaded with 40% drug previously complexed with MeβCD duly characterised (FTIR, DSC, and SEM) was developed for in vivo study. Pharmacokinetic parameters of developed formulation were assessed and compared with oral route of administration. Among various permeation enhancers (PEs), Transcutol P exhibited most enhanced permeation (ER ≈ 1.99) in terms of flux and Q24 compared to control group having. Mean of maximum plasma concentration (Cmax) and area under time-concentration curve (AUC0–72) in Wistar rats (n = 6) for transdermal patch (10 mg/kg) was found to be 70.31 ± 11.2 ng/ml and 2997.29 ± 387.4 ng/ml*h, respectively, and were considerably higher than oral dose of DLX (20 mg/kg and 10 mg/kg). Albeit, T1/2 was higher in case of transdermal delivery, but this was due to sustained behaviour of delivery system. These findings highlight the significance of both inclusion complexation and transdermal delivery of DLX using DIA patch for efficient drug absorption.





Similar content being viewed by others
Data Availability
The datasets supporting study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Marshall JJ, Miwa I. Kinetic difference between hydrolyses of γ-cyclodextrin by human salivary and pancreatic α-amylases. Biochimica et Biophysica Acta (BBA)-Enzymology. 1981;661(1):142–7.
Miller JM, Dahan A. Predicting the solubility–permeability interplay when using cyclodextrins in solubility-enabling formulations: model validation. Int J Pharm. 2012;430(1–2):388–91.
Beig A, Agbaria R, Dahan A. Oral delivery of lipophilic drugs: the tradeoff between solubility increase and permeability decrease when using cyclodextrin-based formulations. PLoS ONE. 2013;8(7): e68237.
Muankaew C, Loftsson T. Cyclodextrin-based formulations: a non-invasive platform for targeted drug delivery. Basic Clin Pharmacol Toxicol. 2018;122(1):46–55.
Yu Y-Q, Yang X, Wu X-F, Fan Y-B. Enhancing permeation of drug molecules across the skin via delivery in nanocarriers: novel strategies for effective transdermal applications. Front Bioeng Biotechnol. 2021;9: 646554.
Sindhu P, Kumar S, Iqbal B, Ali J, Baboota S. Duloxetine loaded-microemulsion system to improve behavioral activities by upregulating serotonin and norepinephrine in brain for the treatment of depression. J Psychiatr Res. 2018;99:83–95.
Hillery AM, Park K. Drug delivery: fundamentals and applications: CRC Press; 2016.
Lobo S, Sachdeva S, Goswami T. Role of pressure-sensitive adhesives in transdermal drug delivery systems. Ther Deliv. 2016;7(1):33–48.
Kumar R, Sinha V, Dahiya L, Sarwal A. Transdermal delivery of duloxetine-sulfobutylether-β-cyclodextrin complex for effective management of depression. Int J Pharm. 2021;594: 120129.
Jung E, Lee EY, Choi H-K, Ban S-J, Choi S-H, Kim JS, et al. Development of drug-in-adhesive patch formulations for transdermal delivery of fluoxetine: in vitro and in vivo evaluations. Int J Pharm. 2015;487(1–2):49–55.
Su Y, Lu W, Fu X, Xu Y, Ye L, Yang J, et al. Formulation and pharmacokinetic evaluation of a drug-in-adhesive patch for transdermal delivery of koumine. AAPS PharmSciTech. 2020;21(8):1–11.
Miranda JCd, Martins TEA, Veiga F, Ferraz HG. Cyclodextrins and ternary complexes: technology to improve solubility of poorly soluble drugs. Braz J Pharm Sci. 2011;47(4):665–81.
Mundhara N, Majumder A, Panda D. Methyl-β-cyclodextrin, an actin depolymerizer augments the antiproliferative potential of microtubule-targeting agents. Sci Rep. 2019;9(1):1–12.
Gotoh K, Kariya R, Alam MM, Matsuda K, Hattori S, Maeda Y, et al. The antitumor effects of methyl-β-cyclodextrin against primary effusion lymphoma via the depletion of cholesterol from lipid rafts. Biochem Biophys Res Commun. 2014;455(3–4):285–9.
Kumar R, Sinha V, Dahiya L, Singh G, Sarwal A. Impact of cyclodextrin derivatives on systemic release of duloxetine HCl via buccal route. Drug Dev Ind Pharm. 2020;46(6):931–45.
Santos PS, Souza LK, Araujo TS, Medeiros JVR, Nunes SC, Carvalho RA, et al. Methyl-β-cyclodextrin inclusion complex with β-caryophyllene: preparation, characterization, and improvement of pharmacological activities. ACS Omega. 2017;2(12):9080–94.
Periasamy R, Kothainayaki S, Rajamohan R, Sivakumar K. Spectral investigation and characterization of host–guest inclusion complex of 4, 4′-methylene-bis (2-chloroaniline) with beta-cyclodextrin. Carbohydr Polym. 2014;114:558–66.
Kumar R, Sinha VR, Dahiya L, Sarwal A. Transdermal delivery of duloxetine-sulfobutylether-β-cyclodextrin complex for effective management of depression. Int J Pharm. 2021;594:120129. https://doi.org/10.1016/j.ijpharm.2020.120129.
Siva S, Li C, Cui H, Meenatchi V, Lin L. Encapsulation of essential oil components with methyl-β-cyclodextrin using ultrasonication: solubility, characterization. DPPH and antibacterial assay Ultrason Sonochem. 2020;64: 104997.
Godin B, Touitou E. Transdermal skin delivery: predictions for humans from in vivo, ex vivo and animal models. Adv Drug Deliv Rev. 2007;59(11):1152–61.
Morimoto Y, Hatanaka T, Sugibayashi K, Omiya H. Prediction of skin permeability of drugs: comparison of human and hairless rat skin. J Pharm Pharmacol. 1992;44(8):634–9.
Morimoto S, Nagata S, Yamaguchi S, Fujino Y. Depth profiling of deuterium implanted in thin films by the elastic recoil detection technique of transmission geometry. Nucl Instrum Methods Phys Res B. 1990;48(1–4):478–81.
de Velde F, de Winter BC, Koch BC, van Gelder T, Mouton JW. Non-linear absorption pharmacokinetics of amoxicillin: consequences for dosing regimens and clinical breakpoints. J Antimicrob Chemother. 2016;71(10):2909–17.
Acknowledgements
Authors are thankful to Cyclolabs, Budapest, Hungary, for providing gratis samples of different cyclodextrin derivatives and department of SAIF/CIL, Panjab University Chandigarh, for carrying out different analyses. Department of Pharmaceutics, NIPER, Mohali is acknowledged for extending spray drying facility.
Funding
This work was supported by University Grants Commission (UGC, India) through award letter (F1-17.1/2014–15/RGNF-2014–15-SC-HAR-68055) sanctioned to first author.
Author information
Authors and Affiliations
Contributions
All authors contributed to study conception and design. Material preparation, data collection, and analysis were performed by Rajiv Kumar and Amita Sarwal. First draft of manuscript was written by Rajiv Kumar and all authors commented on previous versions of manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
All protocols involving laboratory animals are approved from institutional animal ethical committee, Panjab University, Chandigarh.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, R., Sinha, V.R., Dahiya, L. et al. Preclinical Investigation of Transdermal Route for Enhanced Bio-performance of Duloxetine HCl. AAPS PharmSciTech 24, 154 (2023). https://doi.org/10.1208/s12249-023-02607-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02607-7