Abstract
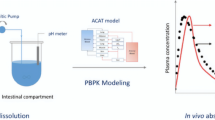
Poorly water-soluble weak base molecules such as cinnarizine often exhibit pH-dependent solubility within the gastrointestinal tract. This means that their solubility can be influenced by the pH of the surrounding environment, and this can affect their oral absorption. The differential pH solubility between the fasted-state stomach and intestine is an important consideration when studying the oral absorption of cinnarizine. Cinnarizine has moderate permeability and is known to exhibit supersaturation and precipitation in fasted-state simulated intestinal fluid (FaSSIF), which can significantly impact its oral absorption. The present work is aimed at studying the precipitation behavior of cinnarizine in FaSSIF using biorelevant in vitro tools and GastroPlus® modeling, to identify the factors contributing to the observed variability in clinical plasma profiles. The study found that cinnarizine demonstrated variable precipitation rates under different bile salt concentrations, which could impact the concentration of the drug available for absorption. The results also showed that a precipitation-integrated modeling approach accurately predicted the mean plasma profiles from the clinical studies. The study concluded that intestinal precipitation may be one of the factors contributing to the observed variability in Cmax but not the AUC of cinnarizine. The study further suggests that the integration of experimental precipitation results representing a wider range of FaSSIF conditions would increase the probability of predicting some of the observed variability in clinical results. This is important for biopharmaceutics scientists, as it can help them evaluate the risk of in vivo precipitation impacting drug and/or drug product performance.







Similar content being viewed by others
Data Availability
All data is available upon request from the corresponding author.
References
Klumpp L, Leigh M, Dressman J. Dissolution behavior of various drugs in different FaSSIF versions. Eur J Pharm Sci. 2020;142: 105138.
Carlert S, Pålsson A, Hanisch G, Von Corswant C, Nilsson C, Lindfors L, et al. Predicting intestinal precipitation—a case example for a basic BCS class II drug. Pharm Res. 2010;27(10):2119–30.
Kambayashi A, Yasuji T, Dressman JB. Prediction of the precipitation profiles of weak base drugs in the small intestine using a simplified transfer (“dumping”) model coupled with in silico modeling and simulation approach. Eur J Pharm Biopharm. 2016;103:95–103.
Kostewicz ES, Aarons L, Bergstrand M, Bolger MB, Galetin A, Hatley O, et al. PBPK models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57:300–21.
Wagner C, Jantratid E, Kesisoglou F, Vertzoni M, Reppas C, Dressman JB. Predicting the oral absorption of a poorly soluble, poorly permeable weak base using biorelevant dissolution and transfer model tests coupled with a physiologically based pharmacokinetic model. Eur J Pharm Biopharm. 2012;82(1):127–38.
Otsuka K, Shono Y, Dressman J. Coupling biorelevant dissolution methods with physiologically based pharmacokinetic modelling to forecast in-vivo performance of solid oral dosage forms. J Pharm Pharmacol. 2013;65(7):937–52.
Kambayashi A, Blume H, Dressman J. Understanding the in vivo performance of enteric coated tablets using an in vitro-in silico-in vivo approach: case example diclofenac. Eur J Pharm Biopharm. 2013;85(3):1337–47.
Berlin M, Przyklenk K-H, Richtberg A, Baumann W, Dressman JB. Prediction of oral absorption of cinnarizine–a highly supersaturating poorly soluble weak base with borderline permeability. Eur J Pharm Biopharm. 2014;88(3):795–806.
Ibrahim F. An enabling formulation of a weakly basic compound guided by Physiologically Based Biopharmaceutics Modeling (PBBM). J Pharm Sci. 2022;2490−2495
Kambayashi A, Blume H, Dressman JB. Predicting the oral pharmacokinetic profiles of multiple-unit (pellet) dosage forms using a modeling and simulation approach coupled with biorelevant dissolution testing: case example diclofenac sodium. Eur J Pharm Biopharm. 2014;87(2):236–43.
Mitra A, Fadda H. Effect of surfactants, gastric emptying, and dosage form on supersaturation of dipyridamole in an in vitro model simulating the stomach and duodenum. Mol Pharm. 2014;11(8):2835–44.
Ruff A, Fiolka T, Kostewicz ES. Prediction of Ketoconazole absorption using an updated in vitro transfer model coupled to physiologically based pharmacokinetic modelling. Eur J Pharm Sci. 2017;100:42–55.
Reppas C, Vertzoni M. Biorelevant in-vitro performance testing of orally administered dosage forms. J Pharm Pharmacol. 2012;64(7):919–30.
Dressman JB, Reppas C. In vitro–in vivo correlations for lipophilic, poorly water-soluble drugs. Eur J Pharm Sci. 2000;11:S73–80.
Nicolaides E, Symillides M, Dressman JB, Reppas C. Biorelevant dissolution testing to predict the plasma profile of lipophilic drugs after oral administration. Pharm Res. 2001;18(3):380–8.
Takano R, Sugano K, Higashida A, Hayashi Y, Machida M, Aso Y, et al. Oral absorption of poorly water-soluble drugs: computer simulation of fraction absorbed in humans from a miniscale dissolution test. Pharm Res. 2006;23(6):1144–56.
Kostewicz ES, Wunderlich M, Brauns U, Becker R, Bock T, Dressman JB. Predicting the precipitation of poorly soluble weak bases upon entry in the small intestine. J Pharm Pharmacol. 2004;56(1):43–51.
Kostewicz ES, Brauns U, Becker R, Dressman JB. Forecasting the oral absorption behavior of poorly soluble weak bases using solubility and dissolution studies in biorelevant media. Pharm Res. 2002;19(3):345.
Ding X, Gueorguieva I, Wesley JA, Burns LJ, Coutant CA. Assessment of in vivo clinical product performance of a weak basic drug by integration of in vitro dissolution tests and physiologically based absorption modeling. AAPS J. 2015;17(6):1395–406.
Moghimipour E, Ameri A, Handali S. Absorption-enhancing effects of bile salts. Molecules. 2015;20(8):14451–73.
Pavlović N, Goločorbin-Kon S, Ðanić M, Stanimirov B, Al-Salami H, Stankov K, et al. Bile acids and their derivatives as potential modifiers of drug release and pharmacokinetic profiles. Front Pharmacol. 2018;9:1283.
Gu CH, Rao D, Gandhi RB, Hilden J, Raghavan K. Using a novel multicompartment dissolution system to predict the effect of gastric pH on the oral absorption of weak bases with poor intrinsic solubility. J Pharm Sci. 2005;94(1):199–208.
Ogata H, Aoyagi N, Kaniwa N, Ejima A, Sekine N, Kitamura M, et al. Gastric acidity dependent bioavailability of cinnarizine from two commercial capsules in healthy volunteers. Int J Pharm. 1986;29(2–3):113–20.
Yamada I, Goda T, Kawata M, Ogawa K. Application of gastric acidity-controlled beagle dog to bioavailability study of cinnarizine. Yakugaku Zasshi. 1990;110(4):280–5.
Nowacka-Krukowska H, Rakowska M, Neubart K, Kobylińska M. High-performance liquid chromatographic assay for cinnarizine in human plasma. Acta pol pharm. 2007;64(5):407–11.
Morrison P, Bradbrook I, Rogers H. Plasma cinnarizine levels resulting from oral administration as capsule or tablet formulation investigated by gas-liquid chromatography. Br J Clin Pharmacol. 1979;7(4):349–52.
Ilie A-R, Griffin BT, Vertzoni M, Kuentz M, Kolakovic R, Prudic-Paus A, et al. Exploring precipitation inhibitors to improve in vivo absorption of cinnarizine from supersaturated lipid-based drug delivery systems. Eur J Pharm Sci. 2021;159: 105691.
Arndt M, Chokshi H, Tang K, Parrott NJ, Reppas C, Dressman JB. Dissolution media simulating the proximal canine gastrointestinal tract in the fasted state. Eur J Pharm Biopharm. 2013;84(3):633–41.
Pedersen PB, Berthelsen R, Rades T, Jørgensen SA, Vilmann P, Bar-Shalom D, et al. Physico-chemical characterization of aspirated human and simulated human gastric fluids to study their influence on the intrinsic dissolution rate of cinnarizine. Int J Pharm. 2022:121856−121862.
Raghuvanshi S, Pathak K. Recent advances in delivery systems and therapeutics of cinnarizine: a poorly water soluble drug with absorption window in stomach. J Drug Deliv. 2014;479246:1−14.
Tanaka Y, Kawakami A, Nanimatsu A, Horio M, Matsuoka J, Wada T, et al. In vivo evaluation of supersaturation/precipitation/re-dissolution behavior of cinnarizine, a lipophilic weak base, in the gastrointestinal tract: the key process of oral absorption. Eur J Pharm Sci. 2017;96:464–71.
Fagerberg JH, Tsinman O, Sun N, Tsinman K, Avdeef A, Bergström CA. Dissolution rate and apparent solubility of poorly soluble drugs in biorelevant dissolution media. Mol Pharm. 2010;7(5):1419–30.
Pepin X, Goetschy M, Abrahmsén-Alami S. Mechanistic models for USP2 dissolution apparatus, including fluid hydrodynamics and sedimentation. J Pharm Sci. 2022;111(1):185–96.
Pepin XJ, Moir AJ, Mann JC, Sanderson NJ, Barker R, Meehan E, et al. Bridging in vitro dissolution and in vivo exposure for acalabrutinib. Part II. A mechanistic PBPK model for IR formulation comparison, proton pump inhibitor drug interactions, and administration with acidic juices. Eur J Pharm Biopharm. 2019;142:435–48.
Bergström CA, Avdeef A. Perspectives in solubility measurement and interpretation. ADMET and DMPK. 2019;7(2):88–105.
Sugano K. Fraction of a dose absorbed estimation for structurally diverse low solubility compounds. Int J Pharm. 2011;405(1–2):79–89.
Hofmann AF, Mysels KJ. Bile acid solubility and precipitation in vitro and in vivo: the role of conjugation, pH, and Ca2+ ions. J Lipid Res. 1992;33(5):617–26.
Boyd BJ, Bergström CA, Vinarov Z, Kuentz M, Brouwers J, Augustijns P, et al. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur J Pharm Sci. 2019;137: 104967.
Chen J, Mosquera-Giraldo LI, Ormes JD, Higgins JD, Taylor LS. Bile salts as crystallization inhibitors of supersaturated solutions of poorly water-soluble compounds. Cryst Growth Des. 2015;15(6):2593–7.
Li N, Mosquera-Giraldo LI, Borca CH, Ormes JD, Lowinger M, Higgins JD, et al. A comparison of the crystallization inhibition properties of bile salts. Cryst Growth Des. 2016;16(12):7286–300.
Litou C, Vertzoni M, Goumas C, Vasdekis V, Xu W, Kesisoglou F, et al. Characteristics of the human upper gastrointestinal contents in the fasted state under hypo-and A-chlorhydric gastric conditions under conditions of typical drug–drug interaction studies. Pharm Res. 2016;33:1399–412.
Aburub A, Chen Y, Chung J, Gao P, Good D, Hansmann S, et al. An IQ consortium perspective on connecting dissolution methods to in vivo performance: analysis of an industrial database and case studies to propose a workflow. AAPS J. 2022;24(3):1–17.
Van Deest B, Fordtran J, Morawski S, Wilson J. Bile salt and micellar fat concentration in proximal small bowel contents of ileectomy patients. J Clin Investig. 1968;47(6):1314–24.
Tack J. the role of bile and pepsin in the pathophysiology and treatment of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2006;24:10–6.
Clarysse S, Tack J, Lammert F, Duchateau G, Reppas C, Augustijns P. Postprandial evolution in composition and characteristics of human duodenal fluids in different nutritional states. J Pharm Sci. 2009;98(3):1177–92.
Petrakis O, Vertzoni M, Angelou A, Kesisoglou F, Bentz K, Goumas K, et al. Identification of key factors affecting the oral absorption of salts of lipophilic weak acids: a case example. J Pharm Pharmacol. 2015;67(1):56–67.
Acknowledgements
The authors would like to thank Ms. Aditi Panchal, Dr. David Harris, and Dr. Marta Venczel for their input, assistance, and support of the work in this manuscript.
Funding
All presented work in this manuscript was performed internally and supported by Sanofi US.
Author information
Authors and Affiliations
Contributions
Conceptualization, data curation, formal analysis, writing (original draft, review, and editing): Siddharth Kesharwani and Fady Ibrahim.
Corresponding author
Ethics declarations
Conflict of Interest
The authors are Sanofi employees and may hold shares and/or stock options in the company.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kesharwani, S.S., Ibrahim, F. A Combined In-Vitro and GastroPlus® Modeling to Study the Effect of Intestinal Precipitation on Cinnarizine Plasma Profile in a Fasted State. AAPS PharmSciTech 24, 121 (2023). https://doi.org/10.1208/s12249-023-02577-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02577-w