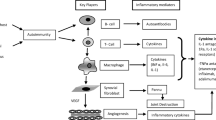
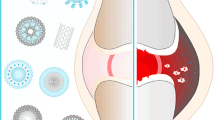
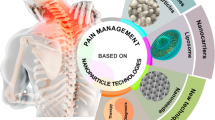
Abstract
Pain disorders are the primary cause of disability nowadays. These disorders, such as rheumatoid arthritis (RA) and osteoarthritis (OA), cause loss of function, joint pain and inflammation and deteriorate the quality of life. The treatment of these inflammatory diseases includes anti-inflammatory drugs administered via intra-articular, topical or oral routes, physical rehabilitation or surgery. Owing to the various side effects these drugs could offer, the novel approaches and nanomaterials have shown potential to manage inflammatory diseases, prolonged half-life of anti-inflammatory drugs, reduced systemic toxicity, provide specific targeting, and refined their bioavailability. This review discusses in brief about the pain pathophysiology and its types. The review summarizes the conventional therapies used to treat pain disorders and the need for novel strategies to overcome the adverse effects of conventional therapies. The review describes the recent advancements in nanotherapeutics for inflammatory diseases using several lipids, polymers and other materials and their excellent efficiency in improving the treatment over conventional therapies. The results of the nanotherapeutic studies inferred that the necessity to use nanocarriers is due to their controlled release, targeting drug delivery to inflamed tissues, low toxicity and biocompatibility. Therefore, it is possible to assert that nanotechnology will emerge as a great tool for advancing the treatment of pain disorders in the near future.
Graphical Abstract






Similar content being viewed by others
Data Availability
The data used to contribute the findings of this review are included within the article.
References
Babaie S, Taghvimi A, Hong J-H, Hamishehkar H, An S, Kim KH. Recent advances in pain management based on nanoparticle technologies. J Nanobiotechnol Internet [Internet] BioMed Central. 2022;20:1–25. https://doi.org/10.1186/s12951-022-01473-y.
Keefe FJ, Kashikar-Zuck S, Opiteck J, Hage E, Dalrymple L, Blumenthal JA. Pain in arthritis and musculoskeletal disorders: the role of coping skills training and exercise interventions. J Orthop Sports Phys Ther. 1996;24:279–90.
National Statistics Text Descriptions | Data and Statistics | Arthritis | CDC [Internet]. [cited 2022 Jul 8]. Available from: https://www.cdc.gov/arthritis/data_statistics/national-statistics-text-version.html. Accessed 08/07/2022.
National Statistics | CDC [Internet]. [cited 2022 Jul 8]. Available from: https://www.cdc.gov/arthritis/data_statistics/national-statistics.html. Accessed 08/07/2022.
Arthritis statistics 2022: What percent of the population has arthritis? [Internet]. [cited 2022 Jul 8]. Available from: https://www.singlecare.com/blog/news/arthritis-statistics/. Accessed 09/07/2022.
Classification of Chronic Pain, Second Edition (Revised) - International Association for the Study of Pain (IASP) [Internet]. [cited 2022 Jul 8]. Available from: https://www.iasp-pain.org/publications/free-ebooks/classification-of-chronic-pain-second-edition-revised/. Accessed 08/07/2022.
Bhansali D, Teng SL, Lee CS, Schmidt BL, Bunnett NW, Leong KW. Nanotechnology for pain management: current and future therapeutic interventions. Nano Today [Internet] Elsevier. 2021;39:101223. https://doi.org/10.1016/j.nantod.2021.101223.
Yam MF, Loh YC, Tan CS, Adam SK, Manan NA, Basir R. General pathways of pain sensation and the major neurotransmitters involved in pain regulation. Int J Mol Sci [Internet]. Multidisciplinary Digital Publishing Institute (MDPI); 2018 [cited 2022 Jul 13];19. Available from: /pmc/articles/PMC6121522/.
Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states – maybe it is all in their head. Best Pract Res Clin Rheumatol [Internet]. NIH Public Access; 2011 [cited 2022 Jul 13];25:141. Available from: /pmc/articles/PMC3220875/.
Sarzi-Puttini P, Atzeni F, Mease PJ. Chronic widespread pain: from peripheral to central evolution. Best Pract Res Clin Rheumatol [Internet]. Best Pract Res Clin Rheumatol; 2011 [cited 2022 Jul 13];25:133–9. Available from: https://pubmed.ncbi.nlm.nih.gov/22094190/.
Tucker-Bartley A, Lemme J, Gomez-Morad A, Shah N, Veliu M, Birklein F, et al. Pain phenotypes in rare musculoskeletal and neuromuscular diseases. Neurosci Biobehav Rev [Internet] Elsevier Ltd. 2021;124:267–90. https://doi.org/10.1016/j.neubiorev.2021.02.009.
Vardeh D, Mannion RJ, Woolf CJ. Toward a mechanism-based approach to pain diagnosis. J Pain [Internet] Elsevier. 2016;17:T50-69. https://doi.org/10.1016/j.jpain.2016.03.001.
Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain [Internet]. Pain; 2008 [cited 2022 Jul 13];136:380–7. Available from: https://pubmed.ncbi.nlm.nih.gov/17888574/.
Bouhassira D. Neuropathic pain: definition, assessment and epidemiology. Rev Neurol (Paris) [Internet]. Elsevier Masson SAS; 2019;175:16–25. Available from: https://doi.org/10.1016/j.neurol.2018.09.016.
Kallakuri S, Singh A, Chen C, Cavanaugh JM. Demonstration of substance P, calcitonin gene-related peptide, and protein gene product 9.5 containing nerve fibers in human cervical facet joint capsules. Spine (Phila Pa 1976) [Internet]. Spine (Phila Pa 1976); 2004 [cited 2022 Jul 14];29:1182–6. Available from: https://pubmed.ncbi.nlm.nih.gov/15167655/. Accessed 14 Jul 2022.
Kim KH, Seo HJ, Abdi S, Huh B. All about pain pharmacology: what pain physicians should know. Korean J Pain. 2020;33:108–20.
Prescott SA, Ratté S. Somatosensation and Pain [Internet]. Conn’s Transl. Neurosci. Elsevier Inc.; 2017. Available from: https://doi.org/10.1016/B978-0-12-802381-5.00037-3.
Geppetti P, Veldhuis NA, Lieu TM, Bunnett NW. G protein-coupled receptors dynamic machines for signaling pain and itch. Neuron [Internet] Elsevier Inc. 2015;88:635–49.
Lin FR, Niparko JK, Ferrucci and L. 基因的改变NIH Public Access. Bone [Internet]. 2014;23:1–7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3624763/pdf/nihms412728.pdf. Accessed 14 Jul 2022.
Asada M. Artificial pain may induce empathy, morality, and ethics in the conscious mind of robots. Philosophies. 2019;4:2–11.
Premkumar L, Sikand P. TRPV1: a target for next generation analgesics. Curr Neuropharmacol. 2008;6:151–63.
Neugebauer V. Chapter 15. Amygdala pain mechanisms. Handb Exp Pharmacol [Internet]. 2015;227:261–84. Available from: http://link.springer.com/https://doi.org/10.1007/978-3-662-46450-2.
Bhoi D, Jain D, Garg R, Iyengar KP, Hoda W, Vaishya R, et al. Complementary and alternative modalities (CAM) for pain management in musculoskeletal diseases (MSDs). J Clin Orthop Trauma [Internet]. Elsevier; 2021 [cited 2022 Jul 14];18:171–80. Available from: http://www.journal-cot.com/article/S0976566221002435/fulltext. Accessed 14 Jul 2022.
Bhoi D, Jain D, Garg R, Iyengar KP, Hoda W, Vaishya R, et al. Complementary and alternative modalities (CAM) for pain management in musculoskeletal diseases (MSDs). J Clin Orthop Trauma [Internet]. Elsevier Ltd; 2021;18:171–80. Available from: https://doi.org/10.1016/j.jcot.2021.04.021.
Emery HM, Bowyer SL, Sisung CE. Rehabilitation of the child with a rheumatic disease. Pediatr Clin North Am [Internet]. Pediatr Clin North Am; 1995 [cited 2022 Jul 20];42:1263–83. Available from: https://pubmed.ncbi.nlm.nih.gov/7567195/. Accessed 20 Jul 2022.
Cakmak A, Bolukbas N. Juvenile rheumatoid arthritis: physical therapy and rehabilitation. South Med J. 2005;98:212–6.
Atkinson TJ, Fudin J. Nonsteroidal antiinflammatory drugs for acute and chronic pain. Phys Med Rehabil Clin N Am [Internet]. Phys Med Rehabil Clin N Am; 2020 [cited 2022 Jul 26];31:219–31. Available from: https://pubmed.ncbi.nlm.nih.gov/32279725/. Accessed 26 Jul 2022.
Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis [Internet]. JKL International LLC; 2018 [cited 2022 Jul 26];9:143. Available from: /pmc/articles/PMC5772852/.
Bach-Rojecky L, Vadunec D, Žunić K, Kurija J, Šipicki S, Gregg R, et al. Continuing war on pain: a personalized approach to the therapy with nonsteroidal anti-inflammatory drugs and opioids. 102217/pme-2018–0116 [Internet]. Future Medicine Ltd London, UK ; 2018 [cited 2022 Jul 26];16:171–84. Available from: https://www.futuremedicine.com/doi/10.2217/pme-2018-0116.
Opioids, Analgesia, and Pain Management | Goodman & Gilman’s: the pharmacological basis of therapeutics, 13e | AccessMedicine | McGraw Hill Medical [Internet]. [cited 2022 Jul 26]. Available from: https://accessmedicine.mhmedical.com/content.aspx?bookid=2189§ionid=170269577. Accessed 26 Jul 2022.
Crockett SD, Greer KB, Heidelbaugh JJ, Falck-Ytter Y, Hanson BJ, Sultan S. American Gastroenterological Association Institute Guideline on the medical management of opioid-induced constipation. Gastroenterology [Internet]. Gastroenterology; 2019 [cited 2022 Jul 26];156:218–26. Available from: https://pubmed.ncbi.nlm.nih.gov/30340754/. Accessed 26 Jul 2022.
Derry S, Cording M, Wiffen PJ, Law S, Phillips T, Moore RA. Pregabalin for pain in fibromyalgia in adults. Cochrane database Syst Rev [Internet]. Cochrane Database Syst Rev; 2016 [cited 2022 Jul 26];9. Available from: https://pubmed.ncbi.nlm.nih.gov/27684492/. Accessed 26 Jul 2022.
Phadke A, Amin P. A recent update on drug delivery systems for pain management. J Pain Palliat Care Pharmacother Internet Taylor & Francis. 2021;35:175–214. https://doi.org/10.1080/15360288.2021.1925386.
Genc H, Nacir B, Duyur Cakit B, Saracoglu M, Erdem HR. The effects of coexisting fibromyalgia syndrome on pain intensity, disability, and treatment outcome in patients with chronic lateral epicondylitis. Pain Med [Internet]. Pain Med; 2012 [cited 2022 Jul 20];13:270–80. Available from: https://pubmed.ncbi.nlm.nih.gov/22222238/. Accessed 20 Jul 2022.
Marianecci C, Di Marzio L, Rinaldi F, Celia C, Paolino D, Alhaique F, et al. Niosomes from 80s to present: the state of the art. Adv Colloid Interface Sci [Internet] Elsevier BV. 2014;205:187–206. https://doi.org/10.1016/j.cis.2013.11.018.
Juch JNS, Maas ET, Ostelo RWJG, George Groeneweg J, Kallewaard JW, Koes BW, et al. Effect of radiofrequency denervation on pain intensity among patients with chronic low back pain: the mint randomized clinical trials. JAMA [Internet]. JAMA; 2017 [cited 2022 Jul 20];318:68–81. Available from: https://pubmed.ncbi.nlm.nih.gov/28672319/. Accessed 20 Jul 2022.
Fletcher D, Stamer UM, Pogatzki-Zahn E, Zaslansky R, Tanase NV, Perruchoud C, et al. Chronic postsurgical pain in Europe: an observational study. Eur J Anaesthesiol [Internet]. Eur J Anaesthesiol; 2015 [cited 2022 Jul 20];32:725–34. Available from: https://pubmed.ncbi.nlm.nih.gov/26241763/. Accessed 20 Jul 2022.
Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet Elsevier B V. 2021;397:2082–97.
Hua S, Wu SY. The use of lipid-based nanocarriers for targeted pain therapies. Front Pharmacol. 2013;4 NOV:1–7.
Ganesan K, Wang Y, Gao F, Liu Q, Zhang C, Li P, et al. Targeting engineered nanoparticles for breast cancer therapy. Pharmaceutics. 2021;13:1–32.
Metselaar JM, Wauben MHM, Wagenaar-Hilbers JPA, Boerman OC, Storm G. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum [Internet]. Arthritis Rheum; 2003 [cited 2022 Aug 2];48:2059–66. Available from: https://pubmed.ncbi.nlm.nih.gov/12847701/. Accessed 2/8/2022.
Wu H, He Y, Wu H, Zhou M, Xu Z, Xiong R, et al. Near-infrared fluorescence imaging-guided focused ultrasound-mediated therapy against rheumatoid arthritis by MTX-ICG-loaded iRGD-modified echogenic liposomes. Theranostics Ivyspring International Publisher. 2020;10:10092–105.
Meka RR, Venkatesha SH, Acharya B, Moudgil KD. Peptide-targeted liposomal delivery of dexamethasone for arthritis therapy. Nanomedicine [Internet]. Future Science Group; 2019 [cited 2022 Aug 3];14:1455. Available from: /pmc/articles/PMC6613046/.
Zheng M, Jia H, Wang H, Liu L, He Z, Zhang Z, et al. Application of nanomaterials in the treatment of rheumatoid arthritis. RSC Adv [Internet]. Royal Society of Chemistry; 2021 [cited 2022 Aug 3];11:7129–37. Available from: https://pubs.rsc.org/en/content/articlehtml/2021/ra/d1ra00328c. Accessed 3/8/2022.
Fan C, Li X, Zhou Y, Zhao Y, Ma S, Li W, et al. Enhanced topical delivery of tetrandrine by ethosomes for treatment of arthritis. Biomed Res Int [Internet]. Hindawi Limited; 2013 [cited 2022 Aug 3];2013. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3766603/. Accessed 3/8/2022.
Kumar Sarwa K, Rudrapal M, Mazumder B. Topical ethosomal capsaicin attenuates edema and nociception in arthritic rats. Drug Deliv [Internet]. Drug Deliv; 2015 [cited 2022 Aug 3];22:1043–52. Available from: https://pubmed.ncbi.nlm.nih.gov/24506573/. Accessed 3/8/2022.
Arora R, Kuhad A, Kaur IP, Chopra K. Curcumin loaded solid lipid nanoparticles ameliorate adjuvant-induced arthritis in rats. Eur J Pain [Internet]. Eur J Pain; 2015 [cited 2022 Aug 4];19:940–52. Available from: https://pubmed.ncbi.nlm.nih.gov/25400173/. Accessed 4/8/2022.
Bhalekar MR, Madgulkar AR, Desale PS, Marium G. Formulation of piperine solid lipid nanoparticles (SLN) for treatment of rheumatoid arthritis. Drug Dev Ind Pharm [Internet]. Drug Dev Ind Pharm; 2017 [cited 2022 Aug 4];43:1003–10. Available from: https://pubmed.ncbi.nlm.nih.gov/28161984/. Accessed 4/8/2022.
Alarifi S, Massadeh S, Al-Agamy M, Al Aamery M, Al Bekairy A, Yassin AE. Enhancement of ciprofloxacin activity by incorporating it in solid lipid nanoparticles. Trop J Pharm Res [Internet]. University of Benin; 2020 [cited 2022 Aug 4];19:909–18. Available from: https://www.ajol.info/index.php/tjpr/article/view/197014. Accessed 4/8/2022.
Wang X, Cao W, Sun C, Wang Y, Wang M, Wu J. Development of pH-sensitive dextran-based methotrexate nanodrug for rheumatoid arthritis therapy through inhibition of JAK-STAT pathways. Int J Pharm. Elsevier; 2022;622:121874.
Tan T, Huang Q, Chu W, Li B, Wu J, Xia Q, et al. Delivery of germacrone (GER) using macrophages-targeted polymeric nanoparticles and its application in rheumatoid arthritis. Drug Deliv [Internet]. Drug Deliv; 2022 [cited 2022 Sep 12];29:692–701. Available from: https://pubmed.ncbi.nlm.nih.gov/35225122/. Accessed 12/9/2022.
Li X, Hou Y, Meng X, Li G, Xu F, Teng L, et al. Folate receptor-targeting mesoporous silica-coated gold nanorod nanoparticles for the synergistic photothermal therapy and chemotherapy of rheumatoid arthritis. RSC Adv [Internet]. RSC Adv; 2021 [cited 2022 Sep 23];11:3567–74. Available from: https://pubmed.ncbi.nlm.nih.gov/35424296/. Accessed 23 Sept 2022.
Simón-Vázquez R, Tsapis N, Lorscheider M, Rodríguez A, Calleja P, Mousnier L, et al. Improving dexamethasone drug loading and efficacy in treating arthritis through a lipophilic prodrug entrapped into PLGA-PEG nanoparticles. Drug Deliv Transl Res [Internet]. Drug Deliv Transl Res; 2022 [cited 2022 Sep 12];12:1270–84. Available from: https://pubmed.ncbi.nlm.nih.gov/34993924/. Accessed 12/9/2022.
Ansari MM, Ahmad A, Kumar A, Alam P, Khan TH, Jayamurugan G, et al. Aminocellulose-grafted-polycaprolactone coated gelatin nanoparticles alleviate inflammation in rheumatoid arthritis: a combinational therapeutic approach. Carbohydr Polym [Internet]. Carbohydr Polym; 2021 [cited 2022 Sep 12];258. Available from: https://pubmed.ncbi.nlm.nih.gov/33593531/. Accessed 12/9/2022.
Zhao M, Zhu T, Chen J, Cui Y, Zhang X, Lee RJ, et al. PLGA/PCADK composite microspheres containing hyaluronic acid-chitosan siRNA nanoparticles: a rational design for rheumatoid arthritis therapy. Int J Pharm [Internet]. Int J Pharm; 2021 [cited 2022 Sep 12];596. Available from: https://pubmed.ncbi.nlm.nih.gov/33493604/. Accessed 12/9/2022.
Paul W, Sharma CP. Inorganic nanoparticles for targeted drug delivery. Biointegration Med Implant Mater Sci Des. Elsevier Ltd.; 2010. p. 204–35.
Li C, Liu R, Song Y, Chen Y, Zhu D, Yu L, et al. Hyaluronic acid hydrogels hybridized with au-triptolide nanoparticles for intraarticular targeted multi-therapy of rheumatoid arthritis. Front Pharmacol [Internet]. Front Pharmacol; 2022 [cited 2022 Sep 22];13. Available from: https://pubmed.ncbi.nlm.nih.gov/35712709/. Accessed 22 Sept 2022.
Li X, Wang H, Zou X, Su H, Li C. Methotrexate-loaded folic acid of solid-phase synthesis conjugated gold nanoparticles targeted treatment for rheumatoid arthritis. Eur J Pharm Sci Elsevier. 2022;170:106101.
Yu M, Jie X, Xu L, Chen C, Shen W, Cao Y, et al. Recent advances in dendrimer research for cardiovascular diseases. Biomacromolecules [Internet]. American Chemical Society; 2015 [cited 2022 Aug 9];16:2588–98. Available from: https://pubs.acs.org/doi/pdf/https://doi.org/10.1021/acs.biomac.5b00979.
Chandrasekar D, Sistla R, Ahmad FJ, Khar RK, Diwan P V. Folate coupled poly(ethyleneglycol) conjugates of anionic poly(amidoamine) dendrimer for inflammatory tissue specific drug delivery. J Biomed Mater Res Part A [Internet]. John Wiley & Sons, Ltd; 2007 [cited 2022 Aug 10];82A:92–103. Available from: https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1002/jbm.a.31122.
Thomas TP, Goonewardena SN, Majoros I, Kotlyar A, Cao Z, Leroueil PR, et al. Folate-targeted nanoparticles show efficacy in the treatment of inflammatory arthritis.
Hayder M, Poupot M, Baron M, Nigon D, Turrin CO, Caminade AM, et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci Transl Med [Internet]. American Association for the Advancement of Science; 2011 [cited 2022 Aug 10];3. Available from: https://www.science.org/doi/https://doi.org/10.1126/scitranslmed.3002212.
Zhao Y-P, Han J-F, Zhang F-Y, Liao T-T, Na R, Yuan X-F, et al. Flexible nano-liposomes-based transdermal hydrogel for targeted delivery of dexamethasone for rheumatoid arthritis therapy. Drug Deliv [Internet]. Taylor & Francis; 2022 [cited 2022 Aug 10];29:2269. Available from: /pmc/articles/PMC9275483/.
Yacoub AS, Ammar HO, Ibrahim M, Mansour SM, Hoffy NM El, El Hoffy NM. Artificial intelligence-assisted development of in situ forming nanoparticles for arthritis therapy via intra-articular delivery. 2022 [cited 2022 Aug 10]; Available from: https://doi.org/10.1080/10717544.2022.2069882.
Li S, Su L, Lv G, Luo W, Kang Y. Ultrasound guided intra-articular injection of triptolide-loaded solid lipid nanoparticle for treatment of antigen-induced arthritis in rabbits. Front Pharmacol [Internet]. Front Pharmacol; 2022 [cited 2022 Aug 10];13. Available from: https://pubmed.ncbi.nlm.nih.gov/35250574/. Accessed 10/8/2022.
Qu B, Wang X lin, Zheng D chong, Mai C tian, Liu Z qiu, Zhou H, et al. Novel treatment for refractory rheumatoid arthritis with total glucosides of paeony and nobiletin codelivered in a self-nanoemulsifying drug delivery system. Acta Pharmacol Sin 2021 438 [Internet]. Nature Publishing Group; 2021 [cited 2022 Aug 10];43:2094–108. Available from: https://www.nature.com/articles/s41401-021-00801-6. Accessed 10/8/2022.
Guo T, Kang X, Ren S, Ouyang X, Chang M, Bernardos A. Construction of a nano-controlled release methotrexate delivery system for the treatment of rheumatoid arthritis by local percutaneous administration. 2021 [cited 2022 Aug 10]; Available from: https://doi.org/10.3390/nano11112812.
Garg NK, Tandel N, Kumar Bhadada S, Tyagi RK, Polito L, Attama A, et al. Nanostructured lipid carrier-mediated transdermal delivery of aceclofenac hydrogel present an effective therapeutic approach for inflammatory diseases. 2021 [cited 2022 Aug 10];12:713616. Available from: www.frontiersin.org. Accessed 10/8/2022.
Sapino S, Chindamo G, Chirio D, Manzoli M, Peira E, Riganti C, et al. Calcium phosphate-coated lipid nanoparticles as a potential tool in bone diseases therapy. Nanomater (Basel, Switzerland) [Internet]. Nanomaterials (Basel); 2021 [cited 2022 Sep 26];11. Available from: https://pubmed.ncbi.nlm.nih.gov/34835747/. Accessed 26 Sept 2022.
Ebada HM, Nasra MM, Nassra RA, Solaiman AA, Abdallah OY. Cationic nanocarrier of rhein based on hydrophobic ion pairing approach as intra-articular targeted regenerative therapy for osteoarthritis. Colloids Surf B Biointerfaces [Internet]. Colloids Surf B Biointerfaces; 2022 [cited 2022 Sep 26];211. Available from: https://pubmed.ncbi.nlm.nih.gov/34942464/. Accessed 2/9/2022.
Goindi S, Kaur A. Evaluation using oxazolone-induced atopic dermatitis in murine model novel flexible vesicles based topical formulation of levocetirizine : in vivo evaluation using oxazolone- induced atopic dermatitis in murine model. 2014.
Rehman M, Madni A, Ihsan A, Khan WS, Khan MI, Mahmood MA, et al. Solid and liquid lipid-based binary solid lipid nanoparticles of diacerein: in vitro evaluation of sustained release, simultaneous loading of gold nanoparticles, and potential thermoresponsive behavior. Int J Nanomedicine [Internet]. Int J Nanomedicine; 2015 [cited 2022 Jul 28];10:2805–14. Available from: https://pubmed.ncbi.nlm.nih.gov/25897224/. Accessed 28 Jul 2022.
Jain A, Mishra SK, Vuddanda PR, Singh SK, Singh R, Singh S. Targeting of diacerein loaded lipid nanoparticles to intra-articular cartilage using chondroitin sulfate as homing carrier for treatment of osteoarthritis in rats. Nanomedicine [Internet]. Nanomedicine; 2014 [cited 2022 Sep 26];10:e1031–40. Available from: https://pubmed.ncbi.nlm.nih.gov/24512762/. Accessed 26 Sept 2022.
Jyothi VGSS, Katta CB, Singothu S, Preeti K, Bhandari V, Singh SB, et al. Analysis of the therapeutic efficacy of meloxicam-loaded solid lipid nanoparticles topical gel in Wistar rats knee osteoarthritis. J Drug Deliv Sci Technol Elsevier. 2022;77:103914.
Jyothi VGSSS, Babu CK, Kumar R, Singh PK, Khatri DK, Singh SB, et al. Meloxicam in combating clinical mastitis: nanotechnology-driven hope and opportunities. J Pharm Bioallied Sci [Internet]. Wolters Kluwer -- Medknow Publications; 2022 [cited 2023 Mar 1];14:121. Available from: /pmc/articles/PMC9728067/.
Jyothi VGSS, Pawar J, Fernandes V, Kumar R, Singh C, Singh SB, et al. Film forming topical dermal spray of meloxicam attenuated pain and inflammation in carrageenan-induced paw oedema in Sprague Dawley rats. J Drug Deliv Sci Technol Elsevier. 2022;70:103195.
Chahal SK, Sodhi RK, Madan J. Duloxetine hydrochloride loaded film forming dermal gel enriched with methylcobalamin and geranium oil attenuates paclitaxel-induced peripheral neuropathy in rats. IBRO Reports Elsevier. 2020;9:85–95.
Moghadam NA, Bagheri F, Eslaminejad MB. Chondroitin sulfate modified chitosan nanoparticles as an efficient and targeted gene delivery vehicle to chondrocytes. Colloids Surf B Biointerfaces [Internet]. Colloids Surf B Biointerfaces; 2022 [cited 2022 Sep 30];219. Available from: https://pubmed.ncbi.nlm.nih.gov/36049252/. Accessed 30 Sept 2022.
Porcello A, Gonzalez-Fernandez P, Jordan O, Allémann E. Nanoforming hyaluronan-based thermoresponsive hydrogels: optimized and tunable functionality in osteoarthritis management. Pharmaceutics [Internet]. Pharmaceutics; 2022 [cited 2022 Sep 30];14. Available from: https://pubmed.ncbi.nlm.nih.gov/35336034/. Accessed 30 Sept 2022.
Xiong W, Lan Q, Liang X, Zhao J, Huang H, Zhan Y, et al. Cartilage-targeting poly(ethylene glycol) (PEG)-formononetin (FMN) nanodrug for the treatment of osteoarthritis. J Nanobiotechnology [Internet]. J Nanobiotechnology; 2021 [cited 2022 Sep 30];19. Available from: https://pubmed.ncbi.nlm.nih.gov/34217311/. Accessed 30 Sept 2022.
Pape E, Parent M, Pinzano A, Sapin-Minet A, Henrionnet C, Gillet P, et al. Rapamycin-loaded Poly(lactic-co-glycolic) acid nanoparticles: preparation, characterization, and in vitro toxicity study for potential intra-articular injection. Int J Pharm [Internet]. Int J Pharm; 2021 [cited 2022 Sep 30];609. Available from: https://pubmed.ncbi.nlm.nih.gov/34662644/. Accessed 30 Sept 2022.
Wang J, Zhang L, Zhu J, Gu J, Wang X, Tao H. Hyaluronic acid modified curcumin-loaded chitosan nanoparticles inhibit chondrocyte apoptosis to attenuate osteoarthritis via upregulation of activator protein 1 and RUNX family transcription factor 2. J Biomed Nanotechnol [Internet]. J Biomed Nanotechnol; 2022 [cited 2022 Sep 30];18:144–57. Available from: https://pubmed.ncbi.nlm.nih.gov/35180907/. Accessed 30 Sept 2022.
Movileanu C, Anghelache M, Turtoi M, Voicu G, Neacsu IA, Ficai D, et al. Folic acid-decorated PEGylated magnetite nanoparticles as efficient drug carriers to tumor cells overexpressing folic acid receptor. Int J Pharm [Internet]. Int J Pharm; 2022 [cited 2022 Oct 6];625. Available from: https://pubmed.ncbi.nlm.nih.gov/35952802/. Accessed 6/10/2022.
Partain BD, Unni M, Rinaldi C, Allen KD. The clearance and biodistribution of magnetic composite nanoparticles in healthy and osteoarthritic rat knees. J Control Release [Internet]. J Control Release; 2020 [cited 2022 Oct 9];321:259–71. Available from: https://pubmed.ncbi.nlm.nih.gov/32004585/. Accessed 9/10/2022.
Marin E, Tapeinos C, Lauciello S, Ciofani G, Sarasua JR, Larrañaga A. Encapsulation of manganese dioxide nanoparticles into layer-by-layer polymer capsules for the fabrication of antioxidant microreactors. Mater Sci Eng C. Elsevier; 2020;117:111349.
Abdel-Aziz MA, Ahmed HMS, El-Nekeety AA, Sharaf HA, Abdel-Aziem SH, Abdel-Wahhab MA. Biosynthesis of gold nanoparticles for the treatment of osteoarthritis alone or in combination with Diacerein® in a rat model. Inflammopharmacology [Internet]. Inflammopharmacology; 2021 [cited 2022 Oct 6];29:705–19. Available from: https://pubmed.ncbi.nlm.nih.gov/34117571/. Accessed 6/10/2022.
Sarkar A, Carvalho E, D’Souza AA, Banerjee R. Liposome-encapsulated fish oil protein-tagged gold nanoparticles for intra-articular therapy in osteoarthritis. Nanomedicine (Lond) [Internet]. Nanomedicine (Lond); 2019 [cited 2022 Oct 6];14:871–87. Available from: https://pubmed.ncbi.nlm.nih.gov/30895865/. Accessed 6/8/2022.
Ren M, Li Y, Zhang H, Li L, He P, Ji P, et al. An oligopeptide/aptamer-conjugated dendrimer-based nanocarrier for dual-targeting delivery to bone. J Mater Chem B [Internet]. J Mater Chem B; 2021 [cited 2022 Oct 9];9:2831–44. Available from: https://pubmed.ncbi.nlm.nih.gov/33704322/. Accessed 6/10/2022.
Geiger BC, Wang S, Padera RF, Grodzinsky AJ, Hammond PT. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci Transl Med [Internet]. Sci Transl Med; 2018 [cited 2022 Oct 9];10. Available from: https://pubmed.ncbi.nlm.nih.gov/30487252/. Accessed 9/10/2022.
Hu Q, Ding B, Yan X, Peng L, Duan J, Yang S, et al. Polyethylene glycol modified PAMAM dendrimer delivery of kartogenin to induce chondrogenic differentiation of mesenchymal stem cells. Nanomedicine [Internet]. Nanomedicine; 2017 [cited 2022 Oct 9];13:2189–98. Available from: https://pubmed.ncbi.nlm.nih.gov/28579434/. Accessed 9/10/2022.
Soliman II, Kandil SM, Abdou EM. Gabapentin–saccharin co-crystals with enhanced physicochemical properties and in vivo absorption formulated as oro-dispersible tablets. Pharm Dev Technol Taylor and Francis Ltd. 2022;25:227–36.
Mota A, Direito R, Carrasco M, … PR-I journal of, 2019 undefined. Combination of hyaluronic acid and PLGA particles as hybrid systems for viscosupplementation in osteoarthritis. Elsevier [Internet]. [cited 2022 Oct 9]; Available from: https://www.sciencedirect.com/science/article/pii/S0378517319300511. Accessed 9/10/2022.
Villamagna IJ, Gordon TN, Hurtig MB, Beier F, Gillies ER. Poly(ester amide) particles for controlled delivery of celecoxib. J Biomed Mater Res A [Internet]. J Biomed Mater Res A; 2019 [cited 2022 Oct 9];107:1235–43. Available from: https://pubmed.ncbi.nlm.nih.gov/30698325/. Accessed 9/10/2022.
T L, M G, W Z. Comparison of therapeutic effects of topical application of diclofenac sodium nanoparticles and conventional placebo on knee osteoarthritis. Cell Mol Biol (Noisy-le-grand) [Internet]. Cell Mol Biol (Noisy-le-grand); 2022 [cited 2022 Oct 9];68:171–8. Available from: https://pubmed.ncbi.nlm.nih.gov/35988175/. Accessed 9/10/2022.
Sangsuwan R, Yik JHN, Owen M, Liu GY, Haudenschild DR, Lewis JS. Intra-articular injection of flavopiridol-loaded microparticles for treatment of post-traumatic osteoarthritis. Acta Biomater [Internet]. Acta Biomater; 2022 [cited 2022 Oct 9];149:347–58. Available from: https://pubmed.ncbi.nlm.nih.gov/35779774/. Accessed 19/10/2022.
Tawfeek GAE, Esaily HA. A novel function of collagen/PCL nanofiber interaction with MSCs in osteoarthritis is potentiation its immunomodulatory effect through increased ICAM expression. Transpl Immunol [Internet]. Transpl Immunol; 2022 [cited 2022 Oct 9];73. Available from: https://pubmed.ncbi.nlm.nih.gov/35569718/. Accessed 9/10/2022.
Zhu C, Han S, Zeng X, Zhu C, Pu Y, Sun Y. Multifunctional thermo-sensitive hydrogel for modulating the microenvironment in Osteoarthritis by polarizing macrophages and scavenging RONS. J Nanobiotechnology [Internet]. J Nanobiotechnology; 2022 [cited 2022 Oct 9];20. Available from: https://pubmed.ncbi.nlm.nih.gov/35526013/. Accessed 9/10/2022.
Xu S, Chang L, Zhao X, Hu Y, Lin Y, Chen Z, et al. Preparation of epigallocatechin gallate decorated Au-Ag nano-heterostructures as NIR-sensitive nano-enzymes for the treatment of osteoarthritis through mitochondrial repair and cartilage protection. Acta Biomater [Internet]. Acta Biomater; 2022 [cited 2022 Oct 9];144:168–82. Available from: https://pubmed.ncbi.nlm.nih.gov/35358735/. Accessed 9/10/2022.
Johnson A, Huang YC, Mao CF, Chen CK, Thomas S, Kuo HP, et al. Protective effect of ethanolic extract of Echinacea purpurea contained nanoparticles on meniscal/ligamentous injury induced osteoarthritis in obese male rats. Sci Rep [Internet]. Sci Rep; 2022 [cited 2022 Oct 9];12. Available from: https://pubmed.ncbi.nlm.nih.gov/35354886/. Accessed 9/10/2022.
Home - ClinicalTrials.gov [Internet]. [cited 2022 Oct 1]. Available from: https://clinicaltrials.gov/. Accessed 1/10/2022.
Funding
The authors acknowledge the financial support offered by Indian Council of Medical Research (ICMR), India to NC through Senior Research Fellowship (45/07/2020-NANO/BMS).
Author information
Authors and Affiliations
Contributions
Nishtha Chaurawal and Mohak Kataria: writing, conceptualization and data collection; Muniramiah Vinod Kumar and Narayan Prasad Mishra: drafting, organization and proof reading; Vijay G. Goni and Kaisar Raza: conceptualization, supervision, drafting and proof reading.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaurawal, N., Kataria, M., Kumar, M.V. et al. Emerging Advances in Nanocarriers Approaches in the Effective Therapy of Pain Related Disorders: Recent Evidence and Futuristic Needs. AAPS PharmSciTech 24, 111 (2023). https://doi.org/10.1208/s12249-023-02567-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02567-y