Abstract
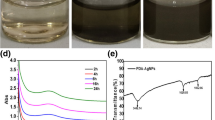
Traditional Asian remedies have mainly employed the macrofungus Ganoderma applanatum, which belongs to the family Ganodermataceae, as a medicinal mushroom due to its high antibacterial and antioxidant activity. Extracts of the fungus can be synthesized into nanoparticles, which are subsequently produced as plaster gels. Synthesized silver nanoparticle-mediated G. applanatum was discovered to have the greatest ability to inhibit bacterial growth in S. epidermidis. When applied to the skin, the prepared plaster gel converted from a gel to a film; thus, both gel and film generation are characteristic of its formulation. The plaster gel that was made was found to be consistent and attractive, and the yellow color had darkened. Its viscosity and pH were appropriate for the application and allowed it to remain on the skin without dripping or reacting with the skin until it dried. A shorter duration for film formation is possible. The film’s tensile was slightly reduced, and it exhibited excellent thermal stability. Decomposition of the generated film occurred at a slower rate, which constrained the polymer chain's ability to move. The semi-crystalline structure was characteristic of the film. It was found that particles were distributed in the film. Rapid release from plaster gel within 4 h was seen, and this was followed by a period of a slowly declining release rate over 12 h. The accurate first-order kinetic used to estimate the release rate of the formulation. The plaster gel demonstrated greater antibacterial activity than the MIC value indicated. The in vivo evaluation was positive and showed no skin irritation. The formulation showed good stability. Therefore, this indicated that the prepared plaster gel is appropriate for topical pharmaceutical delivery and safe for skin application.












Similar content being viewed by others
References
Jogaiah S, Kurjogi M, Abdelrahman M, Hanumanthappa N, Tran L-SP. Ganoderma applanatum-mediated green synthesis of silver nanoparticles: structural characterization, and in vitro and in vivo biomedical and agrochemical properties. Arab J Chem. 2019;12(7):1108–20.
Jeong Y-T, Yang B-K, Jeong S-C, Kim S-M, Song C-H. Ganoderma applanatum: a promising mushroom for antitumor and immunomodulating activity. Phytother Res. 2008;22(5):614–9.
Tsivileva O, Pankratov A, Misin V, Zavyalov A, Volkov V, Tsymbal O, et al. Antioxidant properties of the artist’s conk medicinal mushroom, Ganoderma applanatum (Agaricomycetes), upon cultivation with para-substituted phenolic compounds and tea leaf extracts. Int J Med Mushrooms. 2018;20(6):549–60.
Elkhateeb W, Daba G, El-Dein A, Sheir D, Fayad W, Shaheen M, et al. Insights into the in-vitro hypocholesterolemic, antioxidant, antirotavirus, and anticolon cancer activities of the methanolic extracts of a Japanese lichen, Candelariella vitellina, and a Japanese mushroom Ganoderma applanatum. Egypt Pharmaceut J. 2020;19(1):67–73.
Mohammadifar S, Fallahi Gharaghoz S, Asef Shayan MR, Vaziri A. Comparison between antioxidant activity and bioactive compounds of Ganoderma applanatum (Pers.) Pat. and Ganoderma lucidum (Curt.) P. Karst from Iran. Iran J Plant Physiol. 2020;11(1):3417–24.
Osińska-Jaroszuk M, Jaszek M, Mizerska-Dudka M, Błachowicz A, Rejczak TP, Janusz G, et al. Exopolysaccharide from Ganoderma applanatum as a promising bioactive compound with cytostatic and antibacterial properties. Biomed Res Int. 2014;2014: 743812.
Vijayan R, Joseph S, Mathew B. Eco-friendly synthesis of silver and gold nanoparticles with enhanced antimicrobial, antioxidant, and catalytic activities. IET Nanobiotechnol. 2018;12(6):850–6.
Pathania D, Kumar S, Thakur P, Chaudhary V, Kaushik A, Varma RS, et al. Essential oil-mediated biocompatible magnesium nanoparticles with enhanced antibacterial, antifungal, and photocatalytic efficacies. Sci Rep. 2022;12(1):11431.
Abdellatif AAH, El-Telbany DFA, Zayed G, Al-Sawahli MM. Hydrogel containing PEG-coated fluconazole nanoparticles with enhanced solubility and antifungal activity. J Pharm Innov. 2019;14(2):112–22.
Gupta R, Rai B. In-silico design of nanoparticles for transdermal drug delivery application. Nanoscale. 2018;10(10):4940–51.
Mathur P, Jha S, Ramteke S, Jain NK. Pharmaceutical aspects of silver nanoparticles. Artif Cells Nanomed Biotechnol. 2018;46(sup1):115–26.
Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7(1):17–28.
Gade A, Gaikwad S, Duran N, Rai M. Screening of different species of Phoma for the synthesis of silver nanoparticles. Biotechnol Appl Biochem. 2013;60(5):482–93.
Kathe K, Kathpalia H. Film forming systems for topical and transdermal drug delivery. Asian J Pharm. 2017;12(6):487–97.
Yang S, Yang Y, Cui S, Feng Z, Du Y, Song Z, et al. Chitosan-polyvinyl alcohol nanoscale liquid film-forming system facilitates MRSA-infected wound healing by enhancing antibacterial and antibiofilm properties. Int J Nanomedicine. 2018;13:4987–5002.
Šveikauskaitė I, Briedis V. Effect of film-forming polymers on release of naftifine hydrochloride from nail lacquers. Int J Polym Sci. 2017;2017:1476270.
Kim DW, Kim KS, Seo YG, Lee B-J, Park YJ, Youn YS, et al. Novel sodium fusidate-loaded film-forming hydrogel with easy application and excellent wound healing. Int J Pharm. 2015;495(1):67–74.
Jain N, Singh VK, Chauhan S. A review on mechanical and water absorption properties of polyvinyl alcohol based composites/films. J Mech Behav Mater. 2017;26(5–6):213–22.
Kurakula M, Rao GSNK. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J Drug Deliv Sci Technol. 2020;60: 102046.
Malipeddi VR, Awasthi R, Ghisleni DDM, de Souza Braga M, Kikuchi IS, de Jesus Andreoli Pinto T, et al. Preparation and characterization of metoprolol tartrate containing matrix type transdermal drug delivery system. Drug Deliv Transl Res. 2017;7(1):66–76.
Monton C, Sampaopan Y, Pichayakorn W, Panrat K, Suksaeree J. Herbal transdermal patches made from optimized polyvinyl alcohol blended film: Herbal extraction process, film properties, and in vitro study. J Drug Deliv Sci Technol. 2022;69.
Suksaeree J, Nawathong N, Anakkawee R, Pichayakorn W. Formulation of polyherbal patches based on polyvinyl alcohol and hydroxypropylmethyl cellulose: characterization and in vitro evaluation. AAPS PharmSciTech. 2017;18(7):2427–36.
Dandapat S, Kumar M, Ranjan R, Sinha MP. Ganoderma applanatum extract mediated synthesis of silver nanoparticles. Braz J Pharm Sci. 2022;58: e19173.
Parvekar P, Palaskar J, Metgud S, Maria R, Dutta S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater Investig Dent. 2020;7(1):105–9.
Ahad A, Al-Saleh AA, Al-Mohizea AM, Al-Jenoobi FI, Raish M, Yassin AEB, et al. Pharmacodynamic study of eprosartan mesylate-loaded transfersomes Carbopol® gel under Dermaroller® on rats with methyl prednisolone acetate-induced hypertension. Biomed Pharmacother. 2017;89:177–84.
Sampaopan Y, Suksaeree J. Formulation development and pharmaceutical evaluation of Lysiphyllum strychnifolium topical patches for their anti-inflammatory potential. AAPS PharmSciTech. 2022;23(5):116.
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, et al. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12(3):263–71.
Huang W, Wang Y, Tian W, Cui X, Tu P, Li J, et al. Biosynthesis investigations of terpenoid, alkaloid, and flavonoid antimicrobial agents derived from medicinal plants. Antibiotics. 2022;11(10):1380.
Quester K, Ávalos Borja M, Castro LE. Controllable biosynthesis of small silver nanoparticles using fungal extract. J Biomat Nanobiotechnol. 2016;7:118–25.
Li X, Xu H, Chen Z-S, Chen G. Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomater. 2011;2011: 270974.
Rahi DK, Madhurika B. Biosynthesis of silver nanoparticles by Ganoderma applanatum, evaluation of their antibacterial and antibiotic activity enhancing potential. World J Pharma Pharm. 2015;4(10):1234–47.
Prakash P, Gnanaprakasam P, Emmanuel R, Arokiyaraj S, Saravanan M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf B: Biointerf. 2013;108:255–9.
Thomas R, Janardhanan A, Varghese RT, Mathew EVS, Radhakrishnan EK. Antibacterial properties of silver nanoparticles synthesized by marine Ochrobactrum sp. Braz J Microbiol. 2014;45:1221–7.
Dong Y, Zhu H, Shen Y, Zhang W, Zhang L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLoS ONE. 2019;14(9): e0222322.
Ullah H, Wilfred CD, Shaharun MS. Synthesis of silver nanoparticles using ionic-liquid-based microwave-assisted extraction from Polygonum minus and photodegradation of methylene blue. J Chin Chem Soc. 2017;64(10):1164–71.
Zaheer Z, Rafiuddin. Silver nanoparticles to self-assembled films: Green synthesis and characterization. Colloids Surf B: Biointerf. 2012;90:48-52.
Jyoti K, Baunthiyal M, Singh A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J Rad Res Appl Sci. 2016;9(3):217–27.
Hema JA, Malaka R, Muthukumarasamy NP, Sambandam A, Subramanian S, Sevanan M. Green synthesis of silver nanoparticles using Zea mays and exploration of its biological applications. IET Nanobiotechnol. 2016;10(5):288–94.
Menéndez-Manjón A, Chichkov BN, Barcikowski S. Influence of water temperature on the hydrodynamic diameter of gold nanoparticles from laser ablation. J Phys Chem C. 2010;114(6):2499–504.
Ravichandran V, Vasanthi S, Shalini S, Ali Shah SA, Harish R. Green synthesis of silver nanoparticles using Atrocarpus altilis leaf extract and the study of their antimicrobial and antioxidant activity. Mater Lett. 2016;180:264–7.
Chen LQ, Fang L, Ling J, Ding CZ, Kang B, Huang CZ. Nanotoxicity of silver nanoparticles to red blood cells: size dependent adsorption, uptake, and hemolytic activity. Chem Res Toxicol. 2015;28(3):501–9.
Maneewattanapinyo P, Pichayakorn W, Monton C, Dangmanee N, Wunnakup T, Suksaeree J. Effect of ionic liquid on silver-nanoparticle-complexed Ganoderma applanatum and its topical film formulation. Pharmaceutics. 2023;15(4):1098.
Lambers H, Piessens S, Bloem A, Pronk H, Finkel P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci. 2006;28(5):359–70.
Ranch KM, Maulvi FA, Naik MJ, Koli AR, Parikh RK, Shah DO. Optimization of a novel in situ gel for sustained ocular drug delivery using Box-Behnken design: In vitro, ex vivo, in vivo and human studies. Int J Pharm. 2019;554:264–75.
Felton LA. Mechanisms of polymeric film formation. Int J Pharm. 2013;457(2):423–7.
Salih SI, Jabur AR, Mohammed TA. The effect of PVP addition on the mechanical properties of ternary polymer blends. IOP Conf Ser Mater Sci Eng. 2018;433: 012071.
Arbeiter D, Reske T, Teske M, Bajer D, Senz V, Schmitz K-P, et al. Influence of drug incorporation on the physico-chemical properties of poly(l-lactide) implant coating matrices—a systematic study. Polymers. 2021;13(2):292.
Chandrappa H, Bhajantri RF, Ranjitha, Shwetha, Prarthana N. Simple fabrication of PVA-ATE (Amaranthus tricolor leaves extract) polymer biocomposites: an efficient UV-shielding material for organisms in terrestrial and aquatic ecosystems. Opt Mater. 2020;109:110204.
Pichayakorn W, Maneewattanapinyo P, Panrat K, Monton C, Suksaeree J. Formulation design of oral strip-films based on PVA/PVP polymer blends for nicotine delivery. J Polym Environ. 2022;30(10):4479–91.
Gorain B, Choudhury H, Pandey M, Madheswaran T, Kesharwani P, Tekade RK. Drug–excipient interaction and incompatibilities. In: Tekade RK, editor. Dosage Form Design Parameters: Academic Press; 2018. p. 363–402.
Karak N. Vegetable oil-based polymer composites. In: Karak N, editor. Vegetable Oil-Based Polymers: Woodhead Publishing; 2012. p. 247–70.
Suksaeree J, Thuengernthong A, Pongpichayasiri K, Maneewattanapinyo P, Settharaksa S, Pichayakorn W. Formulation and evaluation of matrix type transdermal patch containing silver nanoparticles. J Polym Environ. 2018;26(12):4369–75.
Singh S, Bharti A, Meena VK. Structural, thermal, zeta potential and electrical properties of disaccharide reduced silver nanoparticles. J Mater Sci: Mater Electron. 2014;25(9):3747–52.
Aziz SB, Marf AS, Dannoun EMA, Brza MA, Abdullah RM. The study of the degree of crystallinity, electrical equivalent circuit, and dielectric properties of polyvinyl alcohol (PVA)-based biopolymer electrolytes. Polymers. 2020;12(10):2184.
Maru S, Gathu L, Mathenge A, Okaru A, Kamau F, Chepkwony H. In vitro drug release studies of metronidazole topical formulations through cellulose membrane. East Cent Afr J Pharm Sci. 2012;15(3):57–62.
Singh Malik D, Mital N, Kaur G. Topical drug delivery systems: a patent review. Expert Opin Ther Pat. 2016;26(2):213–28.
Li W-R, Sun T-L, Zhou S-L, Ma Y-K, Shi Q-S, Xie X-B, et al. A comparative analysis of antibacterial activity, dynamics, and effects of silver ions and silver nanoparticles against four bacterial strains. Int Biodeterior Biodegradation. 2017;123:304–10.
Bruna T, Maldonado-Bravo F, Jara P, Caro N. Silver nanoparticles and their antibacterial applications. Int J Mol Sci. 2021;22(13):7202.
Acknowledgements
The College of Pharmacy and the Research Institute of Rangsit University, Thailand, provided funding for the project (grant number 25/2565). The authors acknowledge Wisawin Hruetrakoon, Kroekkiat Kaewma, Chanchai Suwanlaong, and Jessada Prasomkij as their study assistants. This paper has been proofread in English by the MDPI service, which is financed by Rangsit University's Research Institute.
Author information
Authors and Affiliations
Contributions
Pattwat Maneewattanapinyo and Chaowalit Monton: Paper conception, research, data processing, data analysis, preparation of figures, discussion of results, and paper writing. Wiwat Pichayakorn, Thaniya Wunnakup, and Nattakan Dangmanee: Research, data analysis, data processing, and discussion of results. Jirapornchai Suksaeree: Advising professor, paper conception, research, data processing, data analysis, preparation of graphs and figures, literature review, discussion of results, paper writing, and critical revision and final approval of the article.
Corresponding author
Ethics declarations
Ethics Approval
The Rangsit University Ethics Committee provided its ethical approval (No. RSUERB2022-122, December 6, 2022) for all operations involving healthy human volunteers. All procedures followed to the ICH-GCP, the CIOMS Guideline, the Belmont Report, and the Declaration of Helsinki.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maneewattanapinyo, P., Monton, C., Pichayakorn, W. et al. Plaster Gel Loaded with Silver Nanoparticle-Mediated Ganoderma applanatum: from Fabrication to Evaluation. AAPS PharmSciTech 24, 105 (2023). https://doi.org/10.1208/s12249-023-02566-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02566-z