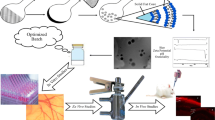
Abstract
Loteprednol etabonate (LE) is a topical corticosteroid that uses inflammatory conditions of the eye. It has a low ocular bioavailability and side effects such as corneal disorder, eye discharge, and ocular discomfort. Therefore, it was decided to select the delivery systems, which are solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and nanoemulsion (NE). Design of experiments (DoE) of SLN, NLC, and NE formulations were formulated by using the quality by design (QbD) approach. Precirol® ATO 5 and oleic acid were used as solid and liquid lipids, respectively, in SLN, NLC, and NE formulations. Physiochemical characterization was performed on the formulations. The optimized formulations’ inflammatory effects have been appraised on human corneal epithelial cells employing the ELISA test. Physicochemical characterization studies and inflammatory effects were appraised. The sizes of optimized formulations of SLN, NLC, and NE were 86.19 nm, 82.38 nm, and 126.35 nm, respectively, with minimum polydispersity. The release behavior of the formulations is composed of both diffusion and erosion. ELISA test results proved that the formulations significantly reduced IL-1 and IL-6 levels (p < 0.05). D-optimal mixture experimental design allowed us to develop the most precise formulations of SLN, NLC, and NE. Furthermore, the optimized formulations could be promising candidates for treating an inflammation-based corneal disease of the eye.
Graphical Abstract






Similar content being viewed by others
Data Availability
The corresponding author will provide the datasets created during and/or analyzed during the current investigation upon reasonable request.
References
Ericson-Neilsen W, Kaye AD. Steroids: pharmacology, complications, and practice delivery issues. Ochsner J. 2014;14(2):203–7.
Pan Q, Xu Q, Boylan NJ, Lamb NW, Emmert DG, Yang JC, et al. Corticosteroid-loaded biodegradable nanoparticles for prevention of corneal allograft rejection in rats. J Control Release. 2015;201:32–40.
Holland EJ, Fingeret M, Mah FS. Use of topical steroids in conjunctivitis: a review of the evidence. Cornea. 2019;38(8):1062–7.
Shimazaki J, Iseda A, Satake Y, Shimazaki-Den S. Efficacy and safety of long-term corticosteroid eye drops after penetrating keratoplasty: a prospective, randomized, clinical trial. Ophthalmology. 2012;119(4):668–73.
Gorantla S, Rapalli VK, Waghule T, Singh PP, Dubey SK, Saha RN, et al. Nanocarriers for ocular drug delivery: current status and translational opportunity. RSC Adv. 2020;10(46):27835–55.
Comstock TL, Sheppard JD. Loteprednol etabonate for inflammatory conditions of the anterior segment of the eye: twenty years of clinical experience with a retrometabolically designed corticosteroid. Expert Opin Pharmacother. 2018;19(4):337–53.
Fong R, Cavet ME, DeCory HH, Vittitow JL. Loteprednol etabonate (submicron) ophthalmic gel 0.38% dosed three times daily following cataract surgery: integrated analysis of two phase III clinical studies. Clin Ophthalmol. 2019;13:1427–38.
Reddy R, Kim SJ. Critical appraisal of ophthalmic ketorolac in treatment of pain and inflammation following cataract surgery. Clin Ophthalmol. 2011;5:751–8.
Balguri SP, Adelli GR, Majumdar S. Topical ophthalmic lipid nanoparticle formulations (SLN, NLC) of indomethacin for delivery to the posterior segment ocular tissues. Eur J Pharm Biopharm. 2016;109:224–35.
Özdemir S, Çelik B, Üner M. Properties and therapeutic potential of solid lipid nanoparticles and nanostructured lipid carriers as promising colloidal drug delivery systems. Materials for Biomedical Engineering: Elsevier; 2019. p. 457–505.
Uner M, Damgali S, Ozdemir S, Celik B. Therapeutic potential of drug delivery by means of lipid nanoparticles: reality or illusion? Curr Pharm Des. 2017;23(43):6573–91.
Müller LJ, Pels E, Vrensen GF. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. 2001;85(4):437–43.
Siekmann B, Bunjes H, Koch MH, Westesen K. Preparation and structural investigations of colloidal dispersions prepared from cubic monoglyceride–water phases. Int J Pharm. 2002;244(1–2):33–43.
Gönüllü Ü, Üner M, Yener G, Fatma Karaman E, Aydoğmuş Z. Formulation and characterization of solid lipid nanoparticles, nanostructured lipid carriers and nanoemulsion of lornoxicam for transdermal delivery. Acta Pharmaceutica. 2015;65(1):1–13.
Deli G, Hatziantoniou S, Nikas Y, Demetzos C. Solid lipid nanoparticles and nanoemulsions containing ceramides: preparation and physicochemical characterization. J Liposome Res. 2009;19(3):180–8.
Özdemir S, Çelik B, Acar ET, Duman G, Üner M. Eplerenone nanoemulsions for treatment of hypertension. Part I: Experimental design for optimization of formulations and physical characterization. J Drug Deliv Sci Technol. 2018;45:357–66.
Dhoot AS, Fernandes GJ, Naha A, Rathnanand M, Kumar L. Design of experiments in pharmaceutical development. Pharm Chem J. 2019;53(8):730–5.
Murray PM, Bellany F, Benhamou L, Bučar D-K, Tabor AB, Sheppard TD. The application of design of experiments (DoE) reaction optimisation and solvent selection in the development of new synthetic chemistry. Org Biomol Chem. 2016;14(8):2373–84.
Souza J, Alves J, Damiani J, Silva M, editors. Design of experiments: its importance in the efficient project management. Proceedings of the 22nd International Conference on Production Research, Iguassu Falls, Brazil. 2013;22:1–5.
López ES, Machado AL, Vidal LB, González-Pizarro R, Silva AD, Souto EB. Lipid nanoparticles as carriers for the treatment of neurodegeneration associated with Alzheimer’s disease and glaucoma: present and future challenges. Curr Pharm Des. 2020;26(12):1235–50.
Badawi N, El-Say K, Attia D, El-Nabarawi M, Elmazar M, Teaima M. Development of pomegranate extract-loaded solid lipid nanoparticles: quality by design approach to screen the variables affecting the quality attributes and characterization. ACS Omega. 2020;5(34):21712–21.
Uner B, Ozdemir S, Tas C, Ozsoy Y, Uner M. Development of lipid nanoparticles for transdermal loteprednol etabonate delivery. J Microencapsul. 2022;39(4):327–40.
Guideline: validation of analytical procedures: textand methodology Q2 (R1). (2005).
Chantaburanan T, Teeranachaideekul V, Chantasart D, Jintapattanakit A, Junyaprasert VB. Effect of binary solid lipid matrix of wax and triglyceride on lipid crystallinity, drug-lipid interaction and drug release of ibuprofen-loaded solid lipid nanoparticles (SLN) for dermal delivery. J Colloid Interface Sci. 2017;504:247–56.
Hu F, Hong Y, Yuan H. Preparation and characterization of solid lipid nanoparticles containing peptide. Int J Pharm. 2004;273(1–2):29–35.
Wagh VD, Apar DU. Cyclosporine a loaded PLGA nanoparticles for dry eye disease: in vitro characterization studies. J Nanotechnol. 2014;683153:1–10.
Han YK, Segall AI. A validated specific stability-indicating RP-HPLC assay method for the determination of loteprednol etabonate in eye drops. J Chromatogr Sci. 2015;53(5):761–6.
Jin L, Wu J, Yuan G, Chen T. In vitro study of the inflammatory cells response to biodegradable Mg-based alloy extract. PLoS ONE. 2018;13(3).
Quantikine®ELISA, For the quantitative determination of human interleukin I (IL-I) and interleukin 6 (IL-6) concentrations in cell culture supernates, serum, and plasma. [Internet]. 14 October 2021. Available from: https://www.bio-techne.com/datasheet-pdf?src=rnd&pdf=d6050.pdf
Üner M. Characterization and imaging of solid lipid nanoparticles and nanostructured lipid carriers. Handbook of nanoparticles: Springer; 2016. p. 117–41.
Hirani A, Lee W, Y, Pathak Y, Sutariya V. Efficacy of loteprednol etabonate drug delivery system in suppression of in vitro retinal pigment epithelium activation. Pharmaceutical Nanotechnology. 2014;2(4):208–16.
Freitas C, Müller RH. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN™) dispersions. Int J Pharm. 1998;168(2):221–9.
Hamdani J, Moës AJ, Amighi K. Physical and thermal characterisation of Precirol® and Compritol® as lipophilic glycerides used for the preparation of controlled-release matrix pellets. Int J Pharm. 2003;260(1):47–57.
Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366(1–2):170–84.
Das S, Ng WK, Kanaujia P, Kim S, Tan RB. Formulation design, preparation and physicochemical characterizations of solid lipid nanoparticles containing a hydrophobic drug: effects of process variables. Colloids Surf, B. 2011;88(1):483–9.
Toameh D. Development of in vitro biocompatibility models of the ocular surface: University of Waterloo; 2020.
Souto E, Wissing S, Barbosa C, Müller R. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm. 2004;278(1):71–7.
Cavalli R, Gasco MR, Chetoni P, Burgalassi S, Saettone MF. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int J Pharm. 2002;238(1–2):241–5.
Qin Q, Luo D, Shi Y, Zhao Q, Chen Y, Wu J, et al. CD25 siRNA induces Treg/Th1 cytokine expression in rat corneal transplantation models. Exp Eye Res. 2016;151:134–41.
Tsai I-L, Tsai C-Y, Kuo L-L, Woung L-C, Ku R-Y, Cheng Y-H. PLGA nanoparticles containing Lingzhi extracts rescue corneal epithelial cells from oxidative damage. Exp Eye Res. 2021;206.
Seyfoddin A, Shaw J, Al-Kassas R. Solid lipid nanoparticles for ocular drug delivery. Drug Delivery. 2010;17(7):467–89.
Başaran E, Demirel M, Sırmagül B, Yazan Y. Cyclosporine-A incorporated cationic solid lipid nanoparticles for ocular delivery. J Microencapsul. 2010;27(1):37–47.
Seyfoddin A, Al-Kassas R. Development of solid lipid nanoparticles and nanostructured lipid carriers for improving ocular delivery of acyclovir. Drug Dev Ind Pharm. 2013;39(4):508–19.
Rathod VR, Shah DA, Dave RH. Systematic implementation of quality-by-design (QbD) to develop NSAID-loaded nanostructured lipid carriers for ocular application: preformulation screening studies and statistical hybrid-design for optimization of variables. Drug Dev Ind Pharm. 2020;46(3):443–55.
Varela-Fernández R, García-Otero X, Díaz-Tomé V, Regueiro U, López-López M, González-Barcia M, et al. Lactoferrin-loaded nanostructured lipid carriers (NLCs) as a new formulation for optimized ocular drug delivery. Eur J Pharm Biopharm. 2022;172:144–56.
Noble S, Goa KL. Loteprednol etabonate. BioDrugs. 1998;10(4):329–39.
Albinus M, Amschler G, Amschler U, von Angerer E, Barthel W, Bauer A, et al. T. In: Albinus M, Amschler G, Amschler U, von Angerer E, Barthel W, Bauer A, et al., editors. Hagers Handbuch der Pharmazeutischen Praxis. Berlin, Heidelberg: Springer Berlin Heidelberg; 1994. p. 765-1129.
Clares B, Calpena AC, Parra A, Abrego G, Alvarado H, Fangueiro JF, et al. Nanoemulsions (NEs), liposomes (LPs) and solid lipid nanoparticles (SLNs) for retinyl palmitate: effect on skin permeation. Int J Pharm. 2014;473(1–2):591–8.
Guzniczak E, Jimenez M, Irwin M, Otto O, Willoughby N, Bridle H. Impact of poloxamer 188 (Pluronic F-68) additive on cell mechanical properties, quantification by real-time deformability cytometry. Biomicrofluidics. 2018;12(4).
Beekes M, Lasch P, Naumann D. Analytical applications of Fourier transform-infrared (FT-IR) spectroscopy in microbiology and prion research. Vet Microbiol. 2007;123(4):305–19.
Mansbridge J, Morhenn V. Tape stripping: a novel noninvasive method using RNA sequences for diagnosing ocular surface diseases of the eye and eyelid. Investigative Ophthalmology & Visual Science. 2020;61(7):392.
Shiyan S, Suryani RP, Mulyani LN, Pratiwi G. Stability study of super saturable catechin-self nano emulsifying drug delivery system as antidiabetic therapy. Biointerface Res Appl Chem. 2022;12:5811–20.
Sheppard JD, Comstock TL, Cavet ME. Impact of the topical ophthalmic corticosteroid loteprednol etabonate on intraocular pressure. Adv Ther. 2016;33(4):532–52.
Bodor N, Loftsson T, Wu W-M. Metabolism, distribution, and transdermal permeation of a soft corticosteroid, loteprednol etabonate. Pharm Res. 1992;9(10):1275–8.
Uner B, Ozdemir S, Yildirim E, Yaba A, Ozsoy Y, Uner M. Loteprednol loaded nanoformulations for corneal delivery: Ex-vivo permeation study, ocular safety assessment and stability studies. J Drug Deliv Sci Technol. 2023;81:1–10.
Conti P, Ronconi G, Caraffa A, Gallenga C, Ross R, Frydas I, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327–31.
Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol. 2021;33(3):127–48.
Venkateswaran N, Bian Y, Gupta PK. Practical guidance for the use of loteprednol etabonate ophthalmic suspension 0.25% in the management of dry eye disease. Clin Ophthalmol. 2022;16:349–55.
Acknowledgements
The authors would not neglect to thank both Yeditepe University Faculty of Genetic and Bioengineering and Yeditepe University Faculty of Chemical Engineering for their unconditional permission analyses throughout the project period. Especially, authors would like to express their deepest gratitude in-person to Dr. Ebru Turkoz Acar, Dr. Manolya Hatipoglu, Dr. Cem Levent Altan, and Beyza Abisoglu.
Author information
Authors and Affiliations
Contributions
Burcu Uner contributed to the investigation, software, methodology, and editing and writing of original manuscript. Samet Ozdemir contributed to the investigation, validation, and review. Yildiz Ozsoy, Melike Uner, and Cetin Tas contributed to the supervision, writing, and review.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uner, B., Ozdemir, S., Tas, C. et al. Loteprednol-Loaded Nanoformulations for Corneal Delivery by Quality-by-Design Concepts: Optimization, Characterization, and Anti-inflammatory Activity. AAPS PharmSciTech 24, 92 (2023). https://doi.org/10.1208/s12249-023-02551-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02551-6