Abstract
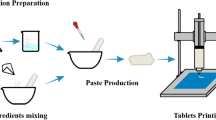
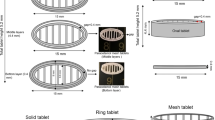
The objective of the present study was to develop digital light processing (DLP) 3D printed sustained release ibuprofen (IBU) tablets using 3D DLP printers for evaluation in in vitro release and in vivo pharmacokinetic studies with their in vitro–in vivo correlation. The resin formulation and printing parameters were optimized using quality by design (QbD) approach, and IBU tablets were printed using DLP printers which works at 385 and 405 nm wavelengths. Our results demonstrated that formulation consisting of polyethylene glycol diacrylate (PEGDA) 700, water, IBU, and riboflavin printed at 40-s bottom layer exposure time and 30-s exposure time produced tablets using both 385 and 405 nm wavelengths. In vitro dissolution studies showed > 70% drug release at the end of 24 h when printed at 405 nm wavelength with no significant difference between tablets printed at 385 nm. In vivo pharmacokinetic evaluation of the optimized 3D printed tablets printed at 405 nm at oral dose of 30 mg/kg in rats showed sustained release of IBU with significantly (p < 0.05) higher Cmax of 30.12 ± 2.45 µg/mL and AUC(0–24 h) of 318.97 ± 16.98 (µg/mL × h) compared to marketed IBU tablet (control). In vivo–in vitro correlation studies showed 80% of drug was absorbed in vivo within 3 h from the pulverized 3D printed tablet, whereas intact 3D tablet showed sustained release of IBU with > 75% IBU release in 24 h in vitro. Overall, IBU tablets fabricated using DLP printing demonstrated sustained release and enhanced systemic absorption with no significant difference in their release profile at different wavelengths.








Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Tan YJN, et al. On-demand fully customizable drug tablets via 3D printing technology for personalized medicine. J Control Release. 2020;322:42–52.
Vaz VM, Kumar L. 3D printing as a promising tool in personalized medicine. AAPS PharmSciTech. 2021;22(1):1–20.
Zhang J, et al. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int J Pharm. 2017;519(1–2):186–97.
Okwuosa TC, et al. Fabricating a shell-core delayed release tablet using dual FDM 3D printing for patient-centred therapy. Pharm Res. 2017;34:427–37.
Clark EA, et al. 3D printing of tablets using inkjet with UV photoinitiation. Int J Pharm. 2017;529(1–2):523–30.
Nukala PK, et al. Abuse deterrent immediate release egg-shaped tablet (Egglets) using 3D printing technology: quality by design to optimize drug release and extraction. AAPS PharmSciTech. 2019;20:1–12.
Nukala PK, et al. Investigating the application of FDM 3D printing pattern in preparation of patient-tailored dosage forms. J 3D Print Med. 2019;3(1):23–37.
Kadry H, et al. Digital light processing (DLP) 3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur J Pharm Sci. 2019;135:60–7.
Stanojević G, et al. Tailoring atomoxetine release rate from DLP 3D-printed tablets using artificial neural networks: influence of tablet thickness and drug loading. Molecules. 2020;26(1):111.
Lin H, et al. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials. 2013;34(2):331–9.
Madzarevic M, et al. Optimization and prediction of ibuprofen release from 3D DLP printlets using artificial neural networks. Pharmaceutics. 2019;11(10):544.
Krkobabić M, et al. Digital light processing (DLP) 3D printing of atomoxetine hydrochloride tablets using photoreactive suspensions. Pharmaceutics. 2020;12(9):833.
Krkobabić M, et al. Hydrophilic excipients in digital light processing (DLP) printing of sustained release tablets: impact on internal structure and drug dissolution rate. Int J Pharm. 2019;572: 118790.
Wang J, et al. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int J Pharm. 2016;503(1–2):207–12.
Gonzalez-Rodriguez M, et al. Channeling agent and drug release from a central core matrix tablet. Drug Dev Ind Pharm. 2001;27(5):439–46.
Madžarević M, Ibrić S. Evaluation of exposure time and visible light irradiation in LCD 3D printing of ibuprofen extended release tablets. Eur J Pharm Sci. 2021;158: 105688.
Samiei N. Recent trends on applications of 3D printing technology on the design and manufacture of pharmaceutical oral formulation: a mini review. Beni-Suef Univ J Basic Appl Sci. 2020;9(1):1–12.
Auriemma G, et al. Additive manufacturing strategies for personalized drug delivery systems and medical devices: fused filament fabrication and semi solid extrusion. Molecules. 2022;27(9):2784.
Czyrski A. Determination of the lipophilicity of ibuprofen, naproxen, ketoprofen, and flurbiprofen with thin-layer chromatography. J Chem. 2019;1–6. https://www.hindawi.com/journals/jchem/2019/3407091/.
Osei-Yeboah F, Sun CC. Validation and applications of an expedited tablet friability method. Int J Pharm. 2015;484(1–2):146–55.
Bagde A, et al. Formulation of topical ibuprofen solid lipid nanoparticle (SLN) gel using hot melt extrusion technique (HME) and determining its anti-inflammatory strength. Drug Deliv Transl Res. 2019;9(4):816–27.
Patil H, et al. Continuous production of fenofibrate solid lipid nanoparticles by hot-melt extrusion technology: a systematic study based on a quality by design approach. AAPS J. 2015;17(1):194–205.
Chowdhury N, et al. Development of hot melt extruded solid dispersion of tamoxifen citrate and resveratrol for synergistic effects on breast cancer cells. AAPS PharmSciTech. 2018;19(7):3287–97.
Wagner JG, Nelson E. Kinetic analysis of blood levels and urinary excretion in the absorptive phase after single doses of drug. J Pharm Sci. 1964;53(11):1392–403.
Kundu A, et al. DLP 3D printed “intelligent” microneedle array (iμNA) for stimuli responsive release of drugs and its in vitro and ex vivo characterization. J Microelectromech Syst. 2020;29(5):685–91.
Fanous M, et al. Development of immediate release (IR) 3D-printed oral dosage forms with focus on industrial relevance. Eur J Pharm Sci. 2020;155: 105558.
Kiani S, et al. Hydrophilicity improvement in polyphenylsulfone nanofibrous filtration membranes through addition of polyethylene glycol. Appl Surf Sci. 2015;359:252–8.
Yagci Y, Jockusch S, Turro NJ. Photoinitiated polymerization: advances, challenges, and opportunities. Macromolecules. 2010;43(15):6245–60.
Endruweit A, Johnson M, Long A. Curing of composite components by ultraviolet radiation: a review. Polym Compos. 2006;27(2):119–28.
Zhang Y, et al. Effect of the synthetic NC-1059 peptide on diffusion of riboflavin across an intact corneal epithelium. Invest Ophthalmol Vis Sci. 2012;53(6):2620–9.
Zanetti-Polzi L, et al. Theoretical modeling of the absorption spectrum of aqueous riboflavin. Chem Phys Lett. 2017;669:119–24.
Shah A, Jung D. Dose-dependent pharmacokinetics of ibuprofen in the rat. Drug Metab Dispos. 1987;15(2):151–4.
Dewland PM, Reader S, Berry P. Bioavailability of ibuprofen following oral administration of standard ibuprofen, sodium ibuprofen or ibuprofen acid incorporating poloxamer in healthy volunteers. BMC Clin Pharmacol. 2009;9(1):1–10.
Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: a review of fundamentals. J Clin Pharmacol. 2002;42(6):620–43.
Yu LX. An integrated model for determining causes of poor oral drug absorption. Pharm Res. 1999;16(12):1883–7.
Siyawamwaya M, et al. 3D printed, controlled release, tritherapeutic tablet matrix for advanced anti-HIV-1 drug delivery. Eur J Pharm Biopharm. 2019;138:99–110.
Choi D-H, Jeong S-H. Multi-layered matrix tablets with various tablet designs and release profiles. J Pharm Investig. 2011;41(5):263–72.
Lemaire V, Belair J, Hildgen P. Structural modeling of drug release from biodegradable porous matrices based on a combined diffusion/erosion process. Int J Pharm. 2003;258(1–2):95–107.
Funding
The authors are thankful to the National Institute on Minority Health and Health Disparities of the National Institutes of Health (grant/award number U54 MD007582) and the NSFCREST Center for Complex Materials Design for Multidimensional Additive Processing (CoManD) (grant/award number:1735968) for providing the funding for this research work.
Author information
Authors and Affiliations
Contributions
Keb Mosley-Kellum: substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work and drafting the work or revising it critically for important intellectual content.
Arvind Bagde: substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work and drafting the work or revising it critically for important intellectual content.
Shawn Spencer: substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work.
Satyanarayan Dev: substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work.
Mandip Singh: final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Ethics approval
The Institutional Animal Care and Use Committee (IACUC) at Florida A&M University, FL approved all animal protocols that were observed in this study (Protocol number: 020-04).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mosley-Kellum, K., Bagde, A., Spencer, S. et al. Development of 3D DLP Printed Sustained Release Ibuprofen Tablets and Their Pharmacokinetic Evaluation in Rats. AAPS PharmSciTech 24, 88 (2023). https://doi.org/10.1208/s12249-023-02544-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02544-5