Abstract
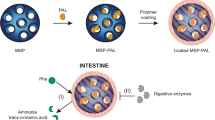
We propose encapsulating phenylalanine ammonia lyase (PAL)-bovine serum albumin (BSA) mixtures as potential oral therapy for the management of phenylketonuria. PAL will metabolize phenylalanine in the gastrointestinal tract while BSA will minimize product inhibition and allow PAL to work at its Vmax. We intend manufacturing microcapsules using spray drying and the proteins will be exposed to heat. In the current pre-formulation studies, we determined the effect of elevated temperatures on the structure and activity of PAL-BSA mixtures and evaluated the stabilizing potential of excipients. Exposure of PAL to 75°C decreased its Vmax. BSA exacerbated the elevated temperature-mediated decrease in PAL Vmax and completely lost the ability to protect PAL from trans cinnamic acid (TCA)-mediated product inhibition. Circular dichroism studies revealed that elevated temperatures did not affect the secondary structure of PAL but decreased BSA α-helicity. Binding experiments showed that elevated temperature-mediated loss in BSA α-helicity was associated with markedly decreased binding and sequestration of TCA, which accounts for the inability of BSA to relieve PAL product inhibition. Sucrose, trehalose, and low concentrations of sodium dodecyl sulfate conferred concentration dependent stabilization of BSA secondary structure against thermal denaturation. The sugars enhanced PAL Vmax, markedly improved TCA binding to BSA, and restored the ability of BSA to relieve PAL product inhibition. PAL-BSA mixtures exposed to elevated temperatures in the presence of sucrose and trehalose exhibited high and constant PAL activity. The results justify inclusion of these sugars in the eventual microcapsule manufacturing process.
Graphical Abstract








Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- BH4:
-
Tetrahydrobiopterin
- CD:
-
Circular dichroism
- g.i.t.:
-
Gastrointestinal tract
- Km:
-
Michaelis constant
- PAH:
-
Phenylalanine hydroxylase
- Phe:
-
Phenylalanine
- PAL:
-
Phenylalanine ammonia lyase
- PKU:
-
Phenylketonuria
- s/o:
-
Solid in oil
- sc:
-
Subcutaneous
- SDS:
-
Sodium dodecyl sulfate
- TCA:
-
Trans cinnamic acid
- V max :
-
Maximum velocity
References
Blau N, van Spronsen FJ, Levy HL. Phenylketonuria Lancet. 2010;376(9750):1417–27.
Feillet F, van Spronsen FJ, MacDonald A, Trefz FK, Demirkol M, Giovannini M, et al. Challenges and pitfalls in the management of phenylketonuria. Pediatrics. 2010;126(2):333–41.
Scriver CR. The PAH gene, phenylketonuria, and a paradigm shift. Hum Mutat. 2007;28(9):831–45.
Vockley J, Andersson HC, Antshel KM, Braverman NE, Burton BK, Frazier DM, et al. Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet Med. 2014;16(2):188–200.
MacDonald A, Gokmen-Ozel H, van Rijn M, Burgard P. The reality of dietary compliance in the management of phenylketonuria. J Inherit Metab Dis. 2010;33(6):665–70.
Trefz FK, Belanger-Quintana A. Sapropterin dihydrochloride: a new drug and a new concept in the management of phenylketonuria. Drugs of today. 2010;46(8):589–600.
Hydery T, Coppenrath VA. A Comprehensive review of pegvaliase, an enzyme substitution therapy for the treatment of phenylketonuria. Drug Target Insights. 2019;13:1177392819857089.
MacDonald MJ, D'Cunha GB. A modern view of phenylalanine ammonia lyase. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2007;85(3):273–82.
Mahan KC, Gandhi MA, Anand S. Pegvaliase: a novel treatment option for adults with phenylketonuria. Curr Med Res Opin. 2019;35(4):647–51.
Besada C, Hakami A, Pillai G, Yetsko K, Truong N, Little T, et al. Preformulation studies with phenylalanine ammonia lyase: essential prelude to a microcapsule formulation for the management of phenylketonuria. J Pharm Sci. 2022;111(7):1857–67.
Putney SD, Burke PA. Improving protein therapeutics with sustained-release formulations. Nat Biotechnol. 1998;16(2):153–7.
Tahara Y, Kamiya N, Goto M. Solid-in-oil dispersion: a novel core technology for drug delivery systems. Int J Pharm. 2012;438(1–2):249–57.
Ameri M, Maa Y-F. Spray drying of biopharmaceuticals: stability and process considerations. Drying Technol. 2006;24:763–8.
Pinto J.T FE, Dieplinger J,Dekner M, Makert C, Nieder M, Paudel A. Progress in spray-drying of protein pharmaceuticals: literature analysis of trends in formulation and process attributes. Drying Technology. 2021;39(11):1415–46.
Habibi-Moini S, D’Mello AP. Evaluation of possible reasons for the low phenylalanine ammonia lyase activity in cellulose nitrate membrane microcapsules. Int J Pharm. 2001;215(1–2):185–96.
Cui JD, Li LL, Bian HJ. Immobilization of cross-linked phenylalanine ammonia lyase aggregates in microporous silica gel. PLoS ONE. 2013;8(11): e80581.
Cui Jd, Ll C, Sp Z, Zhang Yf Su, Zg MG. Hybrid magnetic cross-linked enzyme aggregates of phenylalanine ammonia lyase from Rhodotorula glutinis. PLoS ONE. 2014;9(5): e97221.
Cui J, Liang L, Han C. Stabilization of phenylalanine ammonia lyase from Rhodotorula glutinis by encapsulation in polyethyleneimine-mediated biomimetic silica. Appl Biochem Biotechnol. 2015;176(4):999–1011.
Rees GD, Jones HD. Stability of l-phenylalanine ammonia-lyase in aqueous solution and as the solid state in air and organic solvents. Enzyme Microb Technol. 1996;19(4):282–8.
Good NE, Izawa S. Hydrogen ion buffers. Methods Enzymol. 1972;24:53–68.
Kovacs K, Banoczi G, Varga A, Szabo I, Holczinger A, Hornyanszky G, et al. Expression and properties of the highly alkalophilic phenylalanine ammonia-lyase of thermophilic Rubrobacter xylanophilus. PLoS ONE. 2014;9(1): e85943.
Calabrese JC, Jordan DB, Boodhoo A, Sariaslani S, Vannelli T. Crystal structure of phenylalanine ammonia lyase: multiple helix dipoles implicated in catalysis. Biochemistry. 2004;43(36):11403–16.
Woody RW. Circular dichroism. Methods Enzymol. 1995;246:34–71.
Marini I, Moschini R, Del Corso A, Mura U. Chaperone-like features of bovine serum albumin: a comparison with α-crystallin. Cellular and Molecular Life Sciences CMLS. 2005;62(24):3092–9.
Finn TE, Nunez AC, Sunde M, Easterbrook-Smith SB. Serum albumin prevents protein aggregation and amyloid formation and retains chaperone-like activity in the presence of physiological ligands. J Biol Chem. 2012;287(25):21530–40.
Takeda K, Wada A, Yamamoto K, Moriyama Y, Aoki K. Conformational change of bovine serum albumin by heat treatment. J Protein Chem. 1989;8(5):653–9.
Moriyama Y, Kawasaka Y, Takeda K. Protective effect of small amounts of sodium dodecyl sulfate on the helical structure of bovine serum albumin in thermal denaturation. J Colloid Interface Sci. 2003;257(1):41–6.
Lin VJ, Koenig JL. Raman studies of bovine serum albumin. Biopolymers. 1976;15(1):203–18.
Bischof JC, He X. Thermal stability of proteins. Ann N Y Acad Sci. 2006;1066(1):12–33.
Bian H, Zhang H, Yu Q, Chen Z, Liang H. Studies on the interaction of cinnamic acid with bovine serum albumin. Chem Pharm Bull. 2007;55(6):871–5.
Singh TS, Mitra S. Interaction of cinnamic acid derivatives with serum albumins: a fluorescence spectroscopic study. Spectrochim Acta Part A Mol Biomol Spectrosc. 2011;78(3):942–8.
Nunes NM, Pacheco AFC, Agudelo AJP, da Silva LHM, Pinto MS, Hespanhol MDC, et al. Interaction of cinnamic acid and methyl cinnamate with bovine serum albumin: a thermodynamic approach. Food Chem. 2017;237:525–31.
Paul BK, Samanta A, Guchhait N. Exploring hydrophobic subdomain IIA of the protein bovine serum albumin in the native, intermediate, unfolded, and refolded states by a small fluorescence molecular reporter. J Phys Chem B. 2010;114(18):6183–96.
Tong JHT, Qin A, Sun JZ, Tang BZ. Deciphering the binding behaviours of BSA using ionic AIE-active fluorescent probes. Faraday Discuss. 2017;196:285–303.
Khatun S, Riyazuddeen, Yasmeena,S., Kumar, A., Subbarao,N. Calorimetric, spectroscopic and molecular modelling insight into the interaction of gallic acid with bovine serum albumin. The Journal of Chemical Thermodynamics. 2018;122:85 -94.
Precupas A, Sandu R, Popa VT. Quercetin influence on thermal denaturation of bovine serum albumin. J Phys Chem B. 2016;120(35):9362–75.
Sułkowska A, Maciążek M, Równicka J, Bojko B, Pentak D, Sułkowski WW. Effect of temperature on the methotrexate BSA interaction: spectroscopic study. J Mol Struct. 2007;834:162–9.
Roy AS, Pandey NK, Dasgupta S. Preferential binding of fisetin to the native state of bovine serum albumin: spectroscopic and docking studies. Mol Biol Rep. 2013;40(4):3239–53.
Brown JR. Albumin: structure, function, and uses. Pergamon Press. 1977;Rosenoer, V.M., Oraz, M., and Rotshild, M.A. Eds:27.
Kamerzell TJ, Esfandiary R, Joshi SB, Middaugh CR, Volkin DB. Protein-excipient interactions: mechanisms and biophysical characterization applied to protein formulation development. Adv Drug Deliv Rev. 2011;63(13):1118–59.
Manning MC, Chou DK, Murphy BM, Payne RW, Katayama DS. Stability of protein pharmaceuticals: an update. Pharm Res. 2010;27(4):544–75.
Otzen D. Protein-surfactant interactions: a tale of many states. Biochem Biophys Acta. 2011;1814(5):562–91.
Moriyama Y, Takeda K. Re-formation of the helical structure of human serum albumin by the addition of small amounts of sodium dodecyl sulfate after the disruption of the structure by urea. A comparison with bovine serum albumin. Langmuir. 1999;15(6):2003–8.
Moriyama Y, Watanabe E, Kobayashi K, Harano H, Inui E, Takeda K. Secondary structural change of bovine serum albumin in thermal denaturation up to 130 C and protective effect of sodium dodecyl sulfate on the change. J Phys Chem B. 2008;112(51):16585–9.
Baier S, McClements DJ. Impact of preferential interactions on thermal stability and gelation of bovine serum albumin in aqueous sucrose solutions. J Agric Food Chem. 2001;49(5):2600–8.
Panzica M, Emanuele A, Cordone L. Thermal aggregation of bovine serum albumin in trehalose and sucrose aqueous solutions. J Phys Chem B. 2012;116(39):11829–36.
Shil S, Das N, Sengupta B, Sen P. Sucrose-induced stabilization of domain-ii and overall human serum albumin against chemical and thermal denaturation. ACS Omega. 2018;3(12):16633–42.
Barreca D, Lagana G, Ficarra S, Tellone E, Leuzzi U, Magazu S, et al. Anti-aggregation properties of trehalose on heat-induced secondary structure and conformation changes of bovine serum albumin. Biophys Chem. 2010;147(3):146–52.
Hedoux A, Willart JF, Paccou L, Guinet Y, Affouard F, Lerbret A, et al. Thermostabilization mechanism of bovine serum albumin by trehalose. J Phys Chem B. 2009;113(17):6119–26.
Lavecchia Roberto ZA. Effect of trehalose on thermal stability of bovine serum albumin. Chem Lett. 2010;39:38–9.
Back JF, Oakenfull D, Smith MB. Increased thermal stability of proteins in the presence of sugars and polyols. Biochemistry. 1979;18(23):5191–6.
Kaushik JK, Bhat R. Why is trehalose an exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose. J Biol Chem. 2003;278(29):26458–65.
Lee JC, Timasheff SN. The stabilization of proteins by sucrose. J Biol Chem. 1981;256(14):7193–201.
Arakawa T, Kita Y, Carpenter JF. Protein–solvent interactions in pharmaceutical formulations. Pharm Res. 1991;8(3):285–91.
Arakawa T, Timasheff SN. Stabilization of protein structure by sugars. Biochemistry. 1982;21(25):6536–44.
Liu Y, Bolen DW. The peptide backbone plays a dominant role in protein stabilization by naturally occurring osmolytes. Biochemistry. 1995;34(39):12884–91.
Chang LL, Pikal MJ. Mechanisms of protein stabilization in the solid state. J Pharm Sci. 2009;98(9):2886–908.
Mensink MA, Frijlink HW, van der Voort MK, Hinrichs WL. How sugars protect proteins in the solid state and during drying (review): mechanisms of stabilization in relation to stress conditions. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2017;114:288–95.
Jones LS, Randolph TW, Kohnert U, Papadimitriou A, Winter G, Hagmann ML, et al. The effects of Tween 20 and sucrose on the stability of anti-L-selectin during lyophilization and reconstitution. J Pharm Sci. 2001;90(10):1466–77.
Zhang MZ, Pikal K, Nguyen T, Arakawa T, Prestrelski SJ. The effect of the reconstitution medium on aggregation of lyophilized recombinant interleukin-2 and ribonuclease A. Pharm Res. 1996;13(4):643–6.
Arakawa T, Prestrelski SJ, Kenney WC, Carpenter JF. Factors affecting short-term and long-term stabilities of proteins. Adv Drug Deliv Rev. 2001;46(1–3):307–26.
Allison SD, Chang B, Randolph TW, Carpenter JF. Hydrogen bonding between sugar and protein is responsible for inhibition of dehydration-induced protein unfolding. Arch Biochem Biophys. 1999;365(2):289–98.
Schebor C, Mazzobre MF, Buera MP. Glass transition and time-dependent crystallization behavior of dehydration bioprotectant sugars. Carbohydr Res. 2010;345(2):303–8.
Yadav JK, Prakash V. Thermal stability of α-amylase in aqueous cosolvent systems. J Biosci. 2009;34(3):377–87.
Miroliaei M, Ranjbar B, Naderi-Manesh H, Nemat-Gorgani M. Thermal denaturation of yeast alcohol dehydrogenase and protection of secondary and tertiary structural changes by sugars: CD and fluorescence studies. Enzyme Microb Technol. 2007;40(4):896–901.
Lee J, Ko JH, Lin EW, Wallace P, Ruch F, Maynard HD. Trehalose hydrogels for stabilization of enzymes to heat. Polym Chem. 2015;6(18):3443–8.
Sola-Penna M, Meyer-Fernandes JR. Stabilization against thermal inactivation promoted by sugars on enzyme structure and function: why is trehalose more effective than other sugars? Arch Biochem Biophys. 1998;360(1):10–4.
Acknowledgements
The authors thank Kyle Yetsko for the analyses and plot of the PAL Circular Dichroism data and for helpful discussions.
Funding
This work was supported by funds from Saint Joseph’s University and from the Department of Pharmaceutical Sciences.
Author information
Authors and Affiliations
Contributions
Amerh Alahmadi: performed the experiments, plotted figures, conducted statistical analyses of data, and helped with writing the manuscript.
Anil Dmello: conceptualized studies, supervised experiments, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
Research in our laboratory has received the following patent:
U.S. Patent Application No: 15/495,410, allowed October 16, 2018.
Title: Compositions and methods for the treatment of Phenylketonuria (PKU).
The University of the Sciences has licensed the technology to Abri LLC and the corresponding author owns minority stake in the company.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alahmadi, A., Dmello, A. Detrimental Effects of Elevated Temperatures on the Structure and Activity of Phenylalanine Ammonia Lyase-Bovine Serum Albumin Mixtures and the Stabilizing Potential of Surfactant and Sugars. AAPS PharmSciTech 23, 297 (2022). https://doi.org/10.1208/s12249-022-02446-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02446-y