Abstract
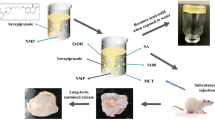
Long-term daily injection of progesterone for the treatment of threatened abortion can be a source of considerable pain to patients. To reduce the frequency of injections and improve patient compliance, a novel injectable phospholipid-based phase separation gel (PPSG) was prepared using small molecular materials such as phospholipids, medium-chain triglycerides (MCTs), and ethanol. Progesterone was loaded into PPSGs to promote rapid gel formation in situ via a sol-gel transformation mechanism, thereby achieving a sustained controlled release. Furthermore, progesterone was distributed in the oil-water interface layer and within the oil phase. Solvent exchange drives phase transitions, and phospholipid vesicle formation and rupture are likely to promote drug release and gel degradation. At a drug loading of 140 mg/mL, a progesterone release of up to 60% could be reached within 9 days according to the release curve in vitro. Pharmacokinetic studies demonstrated that the progesterone-loaded PPSGs released the drug continuously for over 7 days, the half-life was eight times higher than that of progesterone oil solution, and relative bioavailability of up to 184.90% was obtained. Collectively, the sustained release properties for hydrophobic cargos would effectively enhance patient compliance. Moreover, PPSGs are promising drug delivery systems that have high market value and biosafety given the readily accessible and safe excipients.










Similar content being viewed by others
References
Boggess KA, Obstetrics Do, Gynecology UoNC, Chapel Hill, NC, USA., Moss K, Murtha A, Offenbacher S, et al. Antepartum vaginal bleeding, fetal exposure to oral pathogens, and risk for preterm birth at <35 weeks of gestation. Department of Obstetrics and Gynecology, University of North Carolina, Chapel Hill, NC, USA; 2006; 194(4):954-960. https://doi.org/10.1016/j.ajog.2006.02.026.
Verhaegen J, Mello NMV, Gallos ID, Abdel-Aziz M. Accuracy of single progesterone test to predict early pregnancy outcome in women with pain or bleeding: meta-analysis of cohort studies. BMJ Br Med J. 2012;345(7879):18-19.
Esteves SC, Humaidan P, Roque M, Agarwal A. Female infertility and assisted reproductive technology. Andrology and Human Reproduction Clinic, ANDROFERT, Campinas, Brazil - sesteves@androfertcombr Division of Urology, Department of Surgery, University of Campinas (UNICAMP), Campinas, Brazil - sesteves@androfertcombr Faculty of. 2019;61(1):1-2. https://doi.org/10.23736/s0031-0808.18.03553-x.
Szekeres-Bartho J, Wilczynski JR, Basta P, Kalinka J. Role of progesterone and progestin therapy in threatened abortion and preterm labour. Department of Medical Microbiology and Immunology, Medical School, Pecs University, H-7643 Pecs, Hungary. 2008;13(5):1981-90. https://doi.org/10.2741/2817.
Stanczyk FZ, Hapgood JP, Winer S, Mishell DR. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Affiliation <sup class="key">1</sup> Department of Obstetrics and Gynecology, University of Southern California Keck School of Medicine, Livingston Research Building, 1321 North Mission Road, Room 201, Los Angeles, California 90033, US. 2013;34(2):171-208. https://doi.org/10.1210/er.2012-1008.
Simon JA, Robinson DE, Andrews MC, Hildebrand JR, Rocci ML, Blake RE, et al. The absorption of oral micronized progesterone: the effect of food, dose proportionality, and comparison with intramuscular progesterone. Fertil Steril. 1993;60(1):26-33. https://doi.org/10.1016/s0015-0282(16)56031-2.
Renzo GCD, Giardina I, Clerici G, Brillo E, Gerli S. Progesterone in normal and pathological pregnancy. Department of Obstetrics and Gynaecology, Centre for Perinatal and Reproductive Medicine, Santa Maria della Misericordia University Hospital, Perugia, San Sisto, Italy Department of Obstetrics and Gyn. 2016;27(1):35-48. https://doi.org/10.1515/hmbci-2016-0038.
Ginsburg ES, Jellerette-Nolan T, Daftary G, Du Y, Silverberg KM. Patient experience in a randomized trial of a weekly progesterone vaginal ring versus a daily progesterone gel for luteal support after in vitro fertilization. Fertil Steril. 2018;110(6):1101-8(e3). https://doi.org/10.1016/j.fertnstert.2018.07.014.
Ye M, Pan W, Yang X, Zhang X. Research progress of progesterone preparations: a mini review. Department Pharmaceutical, Shenyang Pharmaceutical University, Shenyang 110016, China. 2018;19(10):871-5. https://doi.org/10.2174/1389200219666180427165438.
Tomar L, Tyagi C, Kumar M, Kumar P, Singh H, Choonara YE, et al. In vivo evaluation of a conjugated poly(lactide-ethylene glycol) nanoparticle depot formulation for prolonged insulin delivery in the diabetic rabbit model. Int J Nanomed. 2013;8(1):505-20. https://doi.org/10.2147/ijn.S38011.
Kataoka YW, Makoto 1 Bito, Mika 1 Asai, Jun 1 Katoh, Norito 1. Subcutaneous nodules at progesterone injection sites after fertility treatment. 1 Department of Dermatology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto Japan. 2019;60(2):143-4. https://doi.org/10.1111/ajd.12929.
Veysman B IV, Oshva L. Pneumonitis and eosinophilia after in vitro fertilization treatment. 1 Department of Emergency Medicine, New York University–Bellevue, New York, NY 2 Department of Radiology, New York University–Bellevue, New York, NY. 2005;47(5):472-5. https://doi.org/10.1016/j.annemergmed.2005.12.023.
Wang M, Liu M, Xie T, Zhang B, Gao X. Chitosan-modified cholesterol-free liposomes for improving the oral bioavailability of progesterone. Department of Pharmaceutics, College of Pharmacy, Xinjiang Medical University, Urumqi, China College of Chemistry and Bio-engineering, Yichun University, Yichun, China Affiliated Tumor Hospital of Xinjiang Medical 2017;159:580-5. https://doi.org/10.1016/j.colsurfb.2017.08.028.
Turino LN, Mariano RN, Boimvaser S, Luna JA. In situ-formed microparticles of PLGA from O/W emulsions stabilized with PVA: Encapsulation and controlled release of progesterone(Article). Laboratorio de Química Fina, Instituto de Desarrollo Tecnológico para la Industria Química (INTEC), Universidad Nacional del Litoral (UNL), Ruta Nacional 168 Km 472, Paraje El Pozo, (3000), Santa Fe, Argentina. 2014;9(2):132-40. https://doi.org/10.1007/s12247-014-9180-7.
Ye M, Duan H, Yao L, Fang Y, Zhang X, Dong L, et al. A method of elevated temperatures coupled with magnetic stirring to predict real time release from long acting progesterone PLGA microspheres. 1 Shenyang Pharmaceutical University, 103 Wenhua Road, Shenyang 110016, China 2 Zhejiang University of Technology, 18 Chaowang Road, Zhejiang 310014, China. 2019;14(2):222-32. https://doi.org/10.1016/j.ajps.2018.05.010.
Esposito E, Sguizzato M, Drechsler M, Mariani P, Carducci F, Nastruzzi C, et al. Progesterone lipid nanoparticles: scaling up and in vivo human study. Eur J Pharm Biopharm. 2017;119:437-46. https://doi.org/10.1016/j.ejpb.2017.07.015.
Le Renard P-E, Jordan O, Faes A, Petri-Fink A, Hofmann H, Rüfenacht D, et al. The in vivo performance of magnetic particle-loaded injectable, in situ gelling, carriers for the delivery of local hyperthermia. Biomaterials. 2010;31(4):691-705. https://doi.org/10.1016/j.biomaterials.2009.09.091.
Yang Y, Wang J, Zhang X, Lu W, Zhang Q. A novel mixed micelle gel with thermo-sensitive property for the local delivery of docetaxel. J Controll Release. 2009;135(2):175-82. https://doi.org/10.1016/j.jconrel.2009.01.007.
Pritchard CD, O’Shea TM, Siegwart DJ, Calo E, Anderson DG, Reynolds FM, et al. An injectable thiol-acrylate poly(ethylene glycol) hydrogel for sustained release of methylprednisolone sodium succinate. Biomaterials. 2011;32(2):587-97. https://doi.org/10.1016/j.biomaterials.2010.08.106.
Wu D-Q, Wang T, Lu B, Xu X-D, Cheng S-X, Jiang X-J, et al. Fabrication of supramolecular hydrogels for drug delivery and stem cell encapsulation. Key Laboratory of Biomedical Polymers of Ministry of Education, Department of Chemistry, Wuhan University, Wuhan 430072, China, and Department of Cardiology, Renmin Hospital of Wuhan University, Wuhan 2008;24(18):10306-12. https://doi.org/10.1021/la8006876.
Wu W, Shen J, Banerjee P, Zhou S. Chitosan-based responsive hybrid nanogels for integration of optical pH-sensing, tumor cell imaging and controlled drug delivery. Biomaterials. 2010;31(32):8371-81. https://doi.org/10.1016/j.biomaterials.2010.07.061.
Ehrbar M, Schoenmakers R, Christen EH, Fussenegger M, Weber W. Drug-sensing hydrogels for the inducible release of biopharmaceuticals. 1 Department of Cranio-Maxillofacial Surgery, University Hospital Zurich, Frauenklinikstrasse 24, 8091 Zurich, Switzerland; 2 Department of Biosystems Science and Engineering, ETH Zurich, Mattenstrasse 26, 4058 Basel, Switzerland. 2008;7:800-4. https://doi.org/10.1038/nmat2250.
Salem AK, Rose FRAJ, Oreffo ROC. Porous polymer and cell composites that self-assemble in situ. Adv Mater. 2003;15(3):210-3. https://doi.org/10.1002/adma.200390047.
Packhaeuser CB, Schnieders J, Oster CG, Kissel T. In situ forming parenteral drug delivery systems: an overview. Eur J Pharm Biopharm. 2004;58(2):445-55. https://doi.org/10.1016/j.ejpb.2004.03.003.
Liu F, Urban MW. Recent advances and challenges in designing stimuli-responsive polymers. The University of Southern Mississippi, School of Polymers and High Performance Materials, Hattiesburg, MS 39406, United States. 2010;35(1-2):3-23. https://doi.org/10.1016/j.progpolymsci.2009.10.002.
Kempe S, Mäder K. In situ forming implants — an attractive formulation principle for parenteral depot formulations (Review). J Controll Release. 2012;161(2):668-79. https://doi.org/10.1016/j.jconrel.2012.04.016.
Wang M, Shan F, Zou Y, Sun X, Zhang Z, Fu Y, et al. Pharmacokinetic and pharmacodynamic study of a phospholipid-based phase separation gel for once a month administration of octreotide (Article). J Controll Release. 2016;230:45-56. https://doi.org/10.1016/j.jconrel.2016.03.036.
Qi N, Tang X, Lin X, Gu P, Cai C, Xu H, et al. Sterilization stability of vesicular phospholipid gels loaded with cytarabine for brain implant. Int J Pharm. 2012;427(2):234-41. https://doi.org/10.1016/j.ijpharm.2012.02.008.
Zhong Y, Chen L, Zhang Y, Li W, Sun X, Gong T, et al. Vesicular phospholipid gels using low concentrations of phospholipids for the sustained release of thymopentin: pharmacokinetics and pharmacodynamics. Key Laboratory of Drug Targeting and Drug Delivery Systems, Ministry of Education, West China School of Pharmacy, Sichuan University, Sichuan, People's Republic of China. 2013;68(10):811-5. https://doi.org/10.1691/ph.2013.3008.
Chen T, Gong T, Zhao T, Liu X, Fu Y, Zhang Z, et al. Paclitaxel loaded phospholipid-based gel as a drug delivery system for local treatment of glioma. Int J Pharm. 2017;528(1-2):127-32. https://doi.org/10.1016/j.ijpharm.2017.06.013.
Xiang N, Zhou X, He X, Zhang Y, Zhang J, Zhang Z, et al. An injectable gel platform for the prolonged therapeutic effect of Pitavastatin in the management of hyperlipidemia. 1 Key Laboratory of Drug Targeting and Drug Delivery Systems, Ministry of Education, Department of Pharmaceutical Sciences, West China School of Pharmacy, Sichuan University, Chengdu 610041, China. 2016;105(3):1148-55. https://doi.org/10.1016/j.xphs.2015.12.002.
van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Radboud University Nijmegen, Faculty of Medical School, Geert Grooteplein 9, PO Box 9101, Nijmegen, Netherlands Auckland DHB, 11 Waikato Place, St Johns Park, St Johns, Auckland, New Zealand University of Auckla. 2011;2011(10). https://doi.org/10.1002/14651858.CD009154.pub2.
Unfer V, Casini ML, Marelli G, Costabile L, Gerli S, Renzo GCD. Different routes of progesterone administration and polycystic ovary syndrome: a review of the literature. AGUNCO Obstetrics and Gynecology Centre, Rome, Italy Department of Human Physiology and Pharmacology ʻVittorio Erspamerʼ, University La Sapie. 2005;21(2):119-27. https://doi.org/10.1080/09513590500170049.
Nishikawa M, Ogawa K, Umeki Y. Injectable, self-gelling, biodegradable, and immunomodulatory DNA hydrogel for antigen delivery. J Controll Release. 2014;180(1):25-32. https://doi.org/10.1016/j.jconrel.2014.02.001.
Min-Hyo Ki J-LL, Ko J-Y, Park S-H, Kim J-E, Cho H-J, Park E-S, Kim D-D. A new injectable liquid crystal system for one month delivery of leuprolide. J Controll Release Off J Controll Release Soc. 2014;185(1):62-70. https://doi.org/10.1016/j.jconrel.2014.04.034.
Gurfinkel J, Aserin A, Garti N. Interactions of surfactants in nonionic/anionic reverse hexagonal mesophases and solubilization of α-chymotrypsinogen A. Colloids Surf A Physicochem Eng Asp. 2011;392(1):322-8. https://doi.org/10.1016/j.colsurfa.2011.10.010.
Rosenbaum E, Tavelin S, Johansson LB-Å. A characterisation study on the application of inverted lyotropic phases for subcutaneous drug release. Int J Pharm. 2010;388(1-2):52-7. https://doi.org/10.1016/j.ijpharm.2009.12.032.
Angelico R, Carboni M, Lampis S, Schmidt J, Talmon Y, Monduzzi M, et al. Physicochemical and rheological properties of a novel monoolein-based vesicle gel. a University of Molise (DIAAA) e CSGI, v De Sanctis, I-86100 Campobasso (CB), Italy b Department of Chemical and Geological Science, University of Cagliari, CNBS e CSGI, ss 554 bivio Sestu, I-09042 Monserrato, Italy ; E-mail: murgias@unic. 2013;9(3):921-8. https://doi.org/10.1039/c2sm27215f.
Shchipunov YA. Lecithin organogel - A micellar system with unique properties. Colloids Surf A Physicochem Eng Asp. 2001;183(Special 1):541-54.
Cao Z, Tang X, Zhang Y, Yin T, Gou J, Wang Y, et al. Novel injectable progesterone-loaded nanoparticles embedded in SAIB-PLGA in situ depot system for sustained drug release. Int J Pharm. 2021;607. https://doi.org/10.1016/j.ijpharm.2021.121021.
Salem HF. Sustained-release progesterone nanosuspension following intramuscular injection in ovariectomized rats. Int J Nanomed. 2010;5:943-54. https://doi.org/10.2147/ijn.S12947.
Wu W, Chen H, Shan F, Zhou J, Sun X, Zhang L, et al. A novel doxorubicin-loaded in situ forming gel based high concentration of phospholipid for intratumoral drug delivery. 1 Key Laboratory of Drug Targeting and Drug Delivery Systems, Ministry of Education, Sichuan University, 29 Wangjiang Rd, Chengdu, Sichuan 610041, People’s Republic of China; 2 Faculty of Life Sciences, The University of Manchest. 2014;11(10):3378-85. https://doi.org/10.1021/mp500019p.
Ren T, Cong L, Wang Y, Tang Y, Tian B, Lin X, et al. Lipid emulsions in parenteral nutrition: current applications and future developments. ; 1 Shenyang Pharmaceut Univ, Dept Pharmaceut Sci, Shenyang 110016, Liaoning Provin, Peoples R China ; 2 Xi An Jiao Tong Univ, Sch Mat Sci & Engn, Xian 710049, Peoples R China. 2013;10(11):1533-49. https://doi.org/10.1517/17425247.2013.824874.
Funding
This work was supported by the National Key R&D Program of China (No. 2020YFE0201700), Liaoning Revitalization Talents Program (No. XLYC1908031), and National Mega-project for Innovative Drugs (No. 2019ZX09721001).
Author information
Authors and Affiliations
Contributions
Ning Dong: acquisition, analysis, interpretation, and drafting the work; Lihua Tang: acquisition, analysis, and interpretation; Meihui Zhao: acquisition, analysis; Yingchao Zhang: acquisition, analysis; Yu Zhang: analysis, interpretation; Tian Yin: analysis, interpretation; Haibing He: analysis, interpretation; Jingxin Gou: analysis, interpretation; Yue Yuan: design of the work, analysis, interpretation, and drafting the work. Xing Tang: design of the work, analysis, interpretation, and drafting the work.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, N., Tang, L., Zhao, M. et al. Progesterone Phospholipid Gel for Intramuscular Administration Prepared by In Situ-Phase Separation. AAPS PharmSciTech 23, 294 (2022). https://doi.org/10.1208/s12249-022-02442-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02442-2