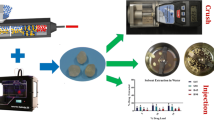
Abstract
Pharmaceutical opioid dosage forms are commonly misused via an oral route in non-manipulated form, i.e., overdose in intact form, or manipulated form, i.e., after crushing the dosage form, and also via the non-oral route in manipulated form, particularly the parenteral or nasal route. To assess the self-regulated anti-overdose property, crushing strength, extractability, and syringeability of the developed drug delivery system by in vitro laboratory studies. Tapentadol HCl drug particulates fabricated using different polymers were assessed for extractability studies in 25 ml of water at room temperature (RT) and at > 90°C. Crushing strength was assessed by grinding the drug particulates in a mortar and pestle and a coffee grinder for 1 min. For syringeability, an attempt was made to withdraw the drug mixture using a 1 ml insulin syringe for 1 min. To assess the self-regulated anti-overdose property, in vitro dissolution testing on a single-capsule per dissolution vessel (normal condition) and four-capsules per dissolution vessel (overdose condition) was performed. POLYOX, Natrosol, and Blanose-containing drug particles retarded drug extraction by > 80% at RT and > 90°C. After 1 min of grinding in a mortar and pestle and a coffee grinder, crushed POLYOX-containing drug particulates were retained at > 99% on the ASTM-170# screen. The attempt to withdraw the viscous mixture of drug formulation prepared with 5 ml of water for 1 min using a 1 ml insulin syringe was unsuccessful. In dissolution studies, more than 90% of the drug was released in normal conditions, and more than 90% of the drug was retarded in overdose conditions. In vitro laboratory studies demonstrate that the developed self-regulated anti-overdose crush-resistant drug delivery system may deter misuse via oral and non-oral routes.
Graphical Abstract






Similar content being viewed by others
Change history
13 October 2022
A Correction to this paper has been published: https://doi.org/10.1208/s12249-022-02433-3
References
Nersesyan H, Slavin KV. Current aproach to cancer pain management: availability and implications of different treatment options. Ther Clin Risk Manag. 2007;3(3):381–400.
Tsang A, von Korff M, Lee S, et al. Common chronic pain conditions in developed and devloping countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9(10):883–91 http://www.sciencedirect.com/science/article/pii/S1526590008005750.
Ambekar A, Agrawal A, Rao R, Mishra AK, Khandelwal SK, Chadda RK on behalf of the group of investigators for the national survey on extent and pattern of substance use in India. Magnitude of Substance Use in India. New Delhi: Ministry of Social Justice and Empowerment, Government of India; 2019.
Opioid overdose crisis, National Institute on Drug Abuse, https://www.drugabuse.gov/drug-topics/opioids/opioid-overdose-crisis
Economic toll of opioid crisis in U.S. Exceed $1 Trillion since 2001. (https://altarum.org/news/economic-toll-opioid-crisis-us-exceeded-1-trillion-2001
Moorman-Li R, Motycka CA, Inge LD, Congdon JM, Hobson S, Pokropski B. A review of abuse-deterrent opioids for chronic nonmalignant pain. Pharmacy and Therapeutics. 2012;37(7):412–8.
Katz NP, Adams EH, Chilcoat H, et al. Challenges in the development of prescription opioid abuse-deterrent formulations. Clin J Pain. 2007;23(8):648–60.
Simon K, Worthy SL, Barnes MC, Tarbell B. Abuse-deterrent formulations: transitioning the pharmaceutical market to improve public health and safety. Therapeutics advances in Drug Safety. 2015;6(2):67–79.
Abuse deterrent opioids – evaluation and labeling, Guidance for Industry, USFDA (https://www.fda.gov/files/drugs/published/Abuse-Deterrent-Opioids-Evaluation-and-Labeling.pdf)
Adler JA, Mallick-Searle T. An overview of abuse-deterrent opioids and recommendations for practical patient care. J Multidiscip Healthc. 2018;11(11):323–32. https://doi.org/10.2147/JMDH.S166915.
Abuse deterrent opioid analgesics. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/abuse-deterrent-opioid-analgesics
Bhandari KH, Talwar N, Abuse deterrent immediate release formulation, PCT patent application, PCT/CA2014/050506, 2013.
Leech RL, Hall Yung RL, Brzeczko AW, Methods and compositions for deterring abuse, US Patent Application, US20110077238A1, 2009.
Huwyler J, Puchkov M. Abuse deterrent pharmaceutical dosage forms, Europe Patent Application, EP3181124A1, 2015.
McKenna WH, Mannion RO, O'Donnell EP, Huang HH. Tamper resistant dosage forms, US Patent Application, US20140030327A1, 2014.
Thompson ER, Thompson ER, Myslinski NR, Kemeny SF. Extruded immediate release abuse deterrent pill, US patent application, US20160199369A1, 2016.
Brzeczko AW, Hollenbeck RG. Methods and compositions for self-regulated release of active pharmaceutical ingredient, US Patent application, US10688184B2, 2020.
Patel JD, Patel SD. Pharmaceutical abuse deterrent composition, US Patent Application number: US20170157055A1, 2019.
Wening K, Geiβler A, Denker J, Barnscheid L. Tamper-resistant dosage form with immediate release and resistance against solvent extraction, US Patent, US9855263B2, 2018.
Patel JD, Patel KJ, Patel SD, Tamper resistant pharmaceutical composition, US Patent Application number: US20180064817A1, 2018.
Patel JD. Pharmaceutical abuse deterrent composition constructed in more than one strengths, US Patent Application number: US20210046014A1, 2021.
Dissolution testing and acceptance criteria for immediate-release solid oral dosage form drug products containing high solubility drug substances, Guidance for Industry, U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) August 2018 Biopharmaceutics. https://www.fda.gov/files/drugs/published/Dissolution-Testing-and-Acceptance-Criteria-for-Immediate-Release-Solid-Oral-Dosage-Form-Drug-Products-Containing-High-Solubility-Drug-Substances-Guidance-for-Industry.pdf
Acknowledgements
The authors acknowledged the support of Pyrrhic Pharma Private Limited, Colorcon, Ashaland, and Evonic for providing gift samples of the materials. The authors are thankful to Prof. Jayesh Patel, Department of English, B.S. Patel Polytechnics, Ganpat University, Mehsana, Gujarat, India—384012, for carefully editing the final manuscript.
Author information
Authors and Affiliations
Contributions
Jayendrakumar Patel: Conceptualization of design of drug delivery system, fabrication, formal analysis, validation of data, writing the first original draft manuscript, and editing. Rakesh Patel: Execution, analytical method and formal analysis, validation of data, and review of the first original draft manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original article has been updated to change the affiliation of author Patel Jayendrakumar.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jayendrakumar, P., Rakesh, P. Self-regulated Anti-Overdose Crush and Extraction-Resistant Drug Delivery System to Combat Opioid Overdose Crisis. AAPS PharmSciTech 23, 265 (2022). https://doi.org/10.1208/s12249-022-02423-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02423-5