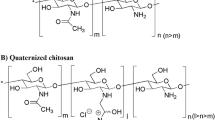
Abstract
β-Cyclodextrin (CD) and chitosan (CS) have attracted great attention due to their unique properties and structures. β-Cyclodextrin-grafted chitosan (CD-CS) has been widely used as a drug carrier to prepare nano-formulations for drug delivery. However, few researches have been conducted to investigate the effect of CD-CS as an excipient on cellular uptake and intestinal absorption. Herein, Caco-2 cells were used to investigate the influence of CD-CS on cellular uptake. The MTT assay showed that CD-CS was non-toxic to Caco-2 cells in concentrations ranging from 15.62 to 125 μg/mL. Confocal laser microscopy and flow cytometry measurements indicated that the uptake ability of Caco-2 cells was significantly enhanced after being treated with CD-CS at a concentration of 31.25 μg/mL or incubation for 0.5 h, and the uptake enhancement gradually increased with increasing CD-CS concentration and incubation time. The Caco-2 monolayer cell model and the everted intestinal sac method were employed to preliminarily explore the mechanism of the improved intestinal absorption. The results demonstrated that CD-CS might open the tight junctions and enhance the clathrin-dependent endocytosis, macro-pinocytosis, and phagocytosis of the intestinal epithelial cells. Such findings can serve as references and inspiration for the design of absorption enhancers.
Graphical abstract










Similar content being viewed by others
References
Wacher VJ, Salphati L, Benet LZ. Active secretion and enterocytic drug metabolism barriers to drug absorption. Adv Drug Deliv Rev. 2001;46(1–3):89–102.
Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41–50.
Duck KA, Connor JR. Iron uptake and transport across physiological barriers. Biometals. 2016;29(4):573–91.
Pifferi G, Santoro P, Pedrani M. Quality and functionality of excipients. Il Farmaco. 1999;54(1–2):1–14.
Pangeni R, Kang S, Jha SK, Subedi L, Park JW. Intestinal membrane transporter-mediated approaches to improve oral drug delivery. Int J Pharm Investig. 2021;51(2):137–58.
Chin YP, Raof SFA, Sinniah S, Lee VS, Mohamad S, Manan NSA. Inclusion complex of Alizarin Red S with β-cyclodextrin: synthesis, spectral, electrochemical and computational studies. J Mol Struct. 2015;1083:236–44.
Barman BK, Barman S, Roy MN. Inclusion complexation between tetrabutylphosphonium methanesulfonate as guest and α-and β-cyclodextrin as hosts investigated by physicochemical methodology. J Mol Liq. 2018;264:80–7.
Vyas A, Saraf S, Saraf S. Cyclodextrin based novel drug delivery systems. J Incl Phenom Macro. 2008;62(1):23–42.
Wang Z-C, Qin CQ, Zhang X, Wang Q, Li RX, Ren DF. Effect of whey protein isolate/chitosan/microcrystalline cellulose/PET multilayer bottles on the shelf life of rosebud beverages. Food Chem. 2021;347: 129006.
Falamarzpour P, Behzad T, Zamani A. Preparation of nanocellulose reinforced chitosan films, cross-linked by adipic acid. Int J Mol Sci. 2017;18(2):396.
Shirosaki Y, Tsuru K, Hayakawa S, Osaka A, Lopes MA, Santos JD, et al. In vitro cytocompatibility of MG63 cells on chitosan-organosiloxane hybrid membranes. Biomaterials. 2005;26(5):485–93.
Jayakumar R, Menon D, Manzoor K, Nair SV, Tamura H. Biomedical applications of chitin and chitosan based nanomaterials-a short review. Carbohydr Polym. 2010;82(2):227–32.
Cardile V, Frasca G, Rizza L, Bonina F, Puglia C, Barge A, et al. Improved adhesion to mucosal cells of water-soluble chitosan tetraalkylammonium salts. Int J Pharm. 2008;362(1–2):88–92.
Yamamoto H, Kuno Y, Sugimoto S, Takeuchi H, Kawashima Y. Surface-modified PLGA nanosphere with chitosan improved pulmonary delivery of calcitonin by mucoadhesion and opening of the intercellular tight junctions. J Control Release. 2005;102(2):373–81.
Ding WY, Zheng SD, Qin Y, Yu F, Bai JW, Cui WQ, et al. Chitosan grafted with β-cyclodextrin: synthesis, characterization, antimicrobial activity, and role as absorbefacient and solubilizer. Front Chem. 2019;6:657–71.
Daimon Y, Izawa H, Kawakami K, Żywicki P, Sakai H, Abe M, et al. Media-dependent morphology of supramolecular aggregates of β-cyclodextrin-grafted chitosan and insulin through multivalent interactions. J Mater Chem B. 2014;2(13):1802–12.
Takechi-Haraya Y, Tanaka K, Tsuji K, Asami Y, Izawa H, Shigenaga A, et al. Molecular complex composed of β-cyclodextrin-grafted chitosan and pH-sensitive amphipathic peptide for enhancing cellular cholesterol efflux under acidic pH. Bioconjug Chem. 2015;26(3):572–81.
Sajomsang W, Nuchuchua O, Gonil P, Saesoo S, Sramala I, Soottitantawat A, et al. Water-soluble β-cyclodextrin grafted with chitosan and its inclusion complex as a mucoadhesive eugenol carrier. Carbohydr Polym. 2012;89(2):623–31.
Izawa H, Haraya YT, Kawakami K. Cyclodextrin–grafted chitosans for pharmaceutical applications. Trends in Glycoscience and Glycotechnology. 2017;29(170):E93–8.
Hou X, Zhang W, He M, Lu Y, Lou K, Gao F. Preparation and characterization of β-cyclodextrin grafted N-maleoyl chitosan nanoparticles for drug delivery. Asian J Pharm. 2017;12(6):558–68.
Campos EVR, Proença PLF, Oliveira JL, Pereira AES, de Morais Ribeiro LN, Fernandes FO, et al. Carvacrol and linalool co-loaded in β-cyclodextrin-grafted chitosan nanoparticles as sustainable biopesticide aiming pest control. Sci Rep. 2018;8(1):1–14.
Zheng SD, Zhang ZY, Ma JX, Qu QW, God’spowe BO, Qin Y, et al. CD-g-CS nanoparticles for enhanced antibiotic treatment of Staphylococcus xylosus infection. Microb Biotechnol. 2022;15(2):535–47.
Gonil P, Sajomsang W, Ruktanonchai UR, Pimpha N, Sramala I, Nuchuchua O, et al. Novel quaternized chitosan containing β-cyclodextrin moiety: synthesis, characterization and antimicrobial activity. Carbohydr Polym. 2011;83(2):905–13.
Wu J, Bu X, Dou L, Fang L, Shen Q. Co-delivery of docetaxel and berbamine by chitosan/sulfobutylether-β-cyclodextrin nanoparticles for enhancing bioavailability and anticancer activities. J Biomed Nanotechnol. 2015;11(10):1847–57.
Vranic S, Boggetto N, Contremoulins V, Mornet S, Reinhardt N, Marano F, et al. Deciphering the mechanisms of cellular uptake of engineered nanoparticles by accurate evaluation of internalization using imaging flow cytometry. Part Fibre Toxicol. 2013;10(1):1–16.
Ujhelyi Z, Fenyvesi F, Váradi J, Fehér P, Kiss T, Veszelka S, et al. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur J Pharm Sci. 2012;47(3):564–73.
Pan F, Han L, Zhang Y, Yu Y, Liu J. Optimization of Caco-2 and HT29 co-culture in vitro cell models for permeability studies. Int J Food Sci Nutr. 2015;66(6):680–5.
Llancalahuen FM, Fuentes JA, Carreño A, Zúñiga C, Páez-Hernández D, Gacitúa M, Polanco R, Preite MD, Arratia-Pérez R, Otero C. New properties of a bioinspired pyridine benzimidazole compound as a novel differential staining agent for endoplasmic reticulum and golgi apparatus in fluorescence live cell imaging. Front Chem. 2018;6:345. https://doi.org/10.3389/fchem.2018.00345.
Yuan H, Chen CY, Chai GH, Du YZ, Hu FQ. Improved transport and absorption through gastrointestinal tract by PEGylated solid lipid nanoparticles. Mol Pharm. 2013;10(5):1865–73.
Zajicek A, Fossler MJ, Barrett JS, Worthington JH, Ternik R, Charkoftaki G, et al. A report from the pediatric formulations task force: perspectives on the state of child-friendly oral dosage forms. AAPS J. 2013;15(4):1072–81.
Wasilewska K, Winnicka K. Ethylcellulose-a pharmaceutical excipient with multidirectional application in drug dosage forms development. Materials. 2019;12(20):3386.
Sun Y, Li L, Xie H, Wang Y, Gao S, Zhang L, et al. Primary studies on construction and evaluation of ion-sensitive in situ gel loaded with paeonol-solid lipid nanoparticles for intranasal drug delivery. Int J Nanomedicine. 2020;15:3137.
Huang M, Khor E, Lim LY. Uptake and cytotoxicity of chitosan molecules and nanoparticles: effects of molecular weight and degree of deacetylation. Pharm Res. 2004;21(2):344–53.
Leroy-Lechat F, Wouessidjewe D, Andreux JP, Puisieux F, Duchêne D. Evaluation of the cytotoxicity of cyclodextrins and hydroxypropylated derivatives. Int J Pharm. 1994;101(1–2):97–103.
Reix N, Parat A, Seyfritz E, Van Der Werf R, Epure V, Ebel N, et al. In vitro uptake evaluation in Caco-2 cells and in vivo results in diabetic rats of insulin-loaded PLGA nanoparticles. Int J Pharm. 2012;437(1–2):213–20.
Jevprasesphant R, Penny J, Attwood D, D’Emanuele A. Transport of dendrimer nanocarriers through epithelial cells via the transcellular route. J Control Release. 2004;97(2):259–67.
Rashki S, Asgarpour K, Tarrahimofrad H, Hashemipour M, Ebrahimi MS, Fathizadeh H, et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr Polym. 2021;251: 117108.
Li X, Uehara S, Sawangrat K, Morishita M, Kusamori K, Katsumi H, et al. Improvement of intestinal absorption of curcumin by cyclodextrins and the mechanisms underlying absorption enhancement. Int J Pharm. 2018;535(1–2):340–9.
Che SY, Yuan JW, Zhang L, Ruan Z, Sun XM, Lu H. Puerarin prevents epithelial tight junction dysfunction induced by ethanol in Caco-2 cell model. J Funct Foods. 2020;73: 104079.
Ranaldi G, Marigliano I, Vespignani I, Perozzi G, Sambuy Y. The effect of chitosan and other polycations on tight junction permeability in the human intestinal Caco-2 cell line. J Nutr Biochem. 2002;13(3):157–67.
Douville NJ, Tung YC, Li R, Wang JD, El-Sayed MEH, Takayama S. Fabrication of two-layered channel system with embedded electrodes to measure resistance across epithelial and endothelial barriers. Anal Chem. 2010;82(6):2505–11.
Kiss L, Hellinger É, Pilbat AM, Kittel Á, Török Z, Füredi A, et al. Sucrose esters increase drug penetration, but do not inhibit P-glycoprotein in Caco-2 intestinal epithelial cells. J Pharm Sci. 2014;103(10):3107–19.
Doi N, Tomita M, Hayashi M. Absorption enhancement of acylcarnitine through changes in tight junction protein in Caco-2 cell monolayers. Drug Metab Pharmacokinet. 2011;26(2):162–70.
Sajeesh S, Bouchemal K, Marsaud V, Vauthier C, Sharma CP. Cyclodextrin complexed insulin encapsulated hydrogel microparticles: an oral delivery system for insulin. J Control Release. 2010;147(3):377–84.
Smith JM, Dornish M, Wood EJ. Involvement of protein kinase C in chitosan glutamate-mediated tight junction disruption. Biomaterials. 2005;26(16):3269–76.
Sieczkarski SB, Whittaker GR. Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis. J Virol. 2002;76(20):10455–64.
Muñoz LE, Boeltz S, Bilyy R, Schauer C, Mahajan A, Widulin N, et al. Neutrophil extracellular traps initiate gallstone formation. Immunity. 2019;51(3):443–50.
Yumoto R, Suzuka S, Oda K, Nagai J, Takano M. Endocytic uptake of FITC-albumin by human alveolar epithelial cell line A549. Drug Metab Pharmacokinet. 2012;27(3):336–43.
Cui S, Qian J, Bo P. Inhibitive effect on phagocytosis of Candida albicans induced by pretreatment with quercetin via actin cytoskeleton interference. J Tradit Chin Med. 2013;33(6):804–9.
Nagai N, Ogata F, Ishii M, Fukuoka Y, Otake H, Nakazawa Y, et al. Involvement of endocytosis in the transdermal penetration mechanism of ketoprofen nanoparticles. Int J Mol Sci. 2018;19(7):2138.
Yang YF, Xu W, Song W, Ye M, Yang XW. Transport of twelve coumarins from Angelicae Pubescentis Radix across a MDCK-pHaMDR cell monolayer-an in vitro model for blood-brain barrier permeability. Molecules. 2015;20(7):11719–32.
Funding
This research was supported by the National Natural Science Foundation of China (No. 82060806), China Postdoctoral Science Foundation (2019BS006), Graduate Education Innovation Program of Guangxi University of Chinese Medicine (YCSZ2022008), Research and Training Project of College Students in Guangxi University of Chinese Medicine (2021DXS09), and Qihuang High-Level Talent Team Cultivation Project of Guangxi University of Chinese Medicine (2021002).
Author information
Authors and Affiliations
Contributions
Linghui Zou, Zhongbin Zhang, and Jinqing Chen performed the experiments and analyzed the data, and Linghui Zou was the major contributor in writing the original manuscript. Xu Yang, Yuyang Li, Jing Tang, Xiaolu Du, and Ling Tang prepared figures for the manuscript. Dan Liang, Jianfang Feng, Xiaoyong Zhu, and Wenya Ding conceptualized, reviewed, and edited the original manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zou, L., Zhang, Z., Chen, J. et al. β-Cyclodextrin-Grafted Chitosan Enhances Intestinal Drug Absorption and Its Preliminary Mechanism Exploration. AAPS PharmSciTech 23, 221 (2022). https://doi.org/10.1208/s12249-022-02380-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02380-z