Abstract
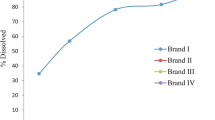
Quantitative evaluation of drug dissolution characteristics based on mathematical models is essential to understand and predict a particular drug release profile. In this study, model-dependent evaluation of the dissolution kinetics of reference and five test products (25-mg, immediate-release (IR) tablets) of an antihypertensive drug, carvedilol, was carried out using the DDSolver® program. The effects of pH (pH 1.2, 4.5, and 6.8) and various media with/without 0.5% (w/v) anionic, cationic, and nonionic surfactants (sodium lauryl sulfate (SLS), hexadecyltrimethylammonium bromide (CTAB), and polysorbate 80) on the dissolution kinetics of the bioequivalent IR products of carvedilol were investigated. The Weibull-1 model was fitted successfully to the dissolution data of all products at pH 1.2 and pH 4.5, as well as in the pH 6.8 medium with CTAB according to the model goodness of fit (r2 = 0.981–0.999, AIC = 14.5–42.6, MSC = 1.99–5.25). Model fitting produced good fits to Gompertz-1 for all products at pH 6.8 without a surfactant (r2 = 0.975–0.998, AIC = 28.3–55, MSC = 2.53–5.82). For pH 6.8 media containing SLS or polysorbate 80, Logistic-2 was fitted successfully to the dissolution data of all products (r2 = 0.974–0.999, AIC = 20.9–52.1, MSC = 1.90–5.69). Overall, the model-dependent analysis of in vitro dissolution data indicated in vitro equivalence of the reference and test products of carvedilol in each medium in terms of kinetic models, suggesting that it would have an important role in developing generic drug products of the BCS class II drug carvedilol.
Graphical Abstract








Similar content being viewed by others
References
National Health Surveillance Agency of Brazil (ANVISA). Resolution RDC no 31. Provides information about the studies of pharmaceutical equivalence and comparative dissolution profile. 2010.
The Center for Drug Evaluation (CDE) of the China National Medical Products Administration (NMPA). Determination and comparison of dissolution curves of ordinary solid preparations guideline. 2016.
European Medicines Agency (EMA). Committee for Medicinal Products for Human Use (CHMP). Guideline on the Investigation of Bioequivalence. Doc.Ref.: CPMP/EWP/QWP/1401/98. Rev. 1/Corr, London. 2010.
U.S. Department of Health and Human Services - Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry: Immediate release solid oral dosage forms: Scale-up and postapproval changes: chemistry, manufacturing, and controls; In Vitro Dissolution Testing and In Vivo Bioequivalence Documentation. 1995.
U.S. Department of Health and Human Services - Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry: Dissolution testing of immediate release solid oral dosage forms. 1997.
U.S. Department of Health and Human Services - Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry: Bioavailability and bioequivalence studies for orally administered drug products, General considerations. 2003.
Health Canada. Guidance Document: Post-Notice of Compliance (NOC) Changes: Quality Document 2019.
Uebbing L, Klumpp L, Webster GK, Löbenberg R. Justification of disintegration testing beyond current FDA criteria using in vitro and in silico models. Drug Des Devel Ther. 2017;11:1163–74 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5395276/.
Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20. https://doi.org/10.1023/A:1016212804288.
Vogelpoel H, Welink J, Amidon GL, Junginger HE, Midha KK, Möller H, Olling M, Shah VP, Barends DM. Biowaiver monographs for immediate release solid oral dosage forms based on biopharmaceutics classification system (BCS) literature data: verapamil hydrochloride, propranolol hydrochloride, and atenolol. J Pharm Sci. 2004;93:1945–56. https://doi.org/10.1002/jps.20131.
Potthast H, Dressman JB, Junginger HE, Midha KK, Oeser H, Shah VP, Vogelpoel H, Barends DM. Biowaiver monographs for immediate release solid oral dosage forms: ibuprofen. J Pharm Sci. 2005;94:2121–31. https://doi.org/10.1002/jps.20444.
Chuasuwa B, Binjesoh V, Polli JE, Zhang H, Amidon GL, Junginger HE, Midha KK, Shah VP, Stavchansky S, Dressman JB, Barends DM. Biowaiver monographs for immediate release solid oral dosage forms: diclofenac sodium and diclofenac potassium. J Pharm Sci. 2009;98:1206–19. https://doi.org/10.1002/jps.21525.
Shohin IE, Kulinich JI, Ramenskaya GV, Abrahamsson B, Kopp S, Langguth P, Polli JE, Shah VP, Groot DW, Barends DM, Dressman JB. Biowaiver monographs for immediate-release solid oral dosage forms: ketoprofen. J Pharm Sci. 2012;101:3593–603. https://doi.org/10.1002/jps.23233.
Shohin IE, Kulinich JI, Ramenskaya GV, Abrahamsson B, Kopp S, Langguth P, Polli JE, Shah VP, Groot DW, Barends DM, Dressman JB. Biowaiver monographs for immediate release solid oral dosage forms: piroxicam. J Pharm Sci. 2014;103:367–77. https://doi.org/10.1002/jps.23799.
Hanif M, Shoaib MH, Yousuf RI, Zafar F. Development of in vitro - in vivo correlations for newly optimized nimesulide formulations. PLoS ONE 2018;13(8):e0203123. https://doi.org/10.1371/journal.pone.0203123.
Gonzalez-Alvarez I, Bermejo M, Tsume Y, Ruiz-Picazo A, Gonzalez-Alvarez M, Hens B, Garcia-Arieta A, Amidon GE, Amidon GL. An in vivo predictive dissolution methodology (iPD methodology) with a BCS class IIb drug can predict the in vivo bioequivalence results: etoricoxib products. Pharmaceutics. 2021;13(507):1–12. https://doi.org/10.3390/pharmaceutics13040507.
Ibarra M, Valiante C, Sopeña P, Schiavo A, Lorier M, Vázquez M, Fagiolino P. Integration of in vitro biorelevant dissolution and in silico PBPK model of carvedilol to predict bioequivalence of oral drug products. Eur J Pharm Sci. 2018;118:176–82. https://doi.org/10.1016/j.ejps.2018.03.032.
Al-Tabakha MM, Alomar MJ. In vitro dissolution and in silico modeling shortcuts in bioequivalence testing. Pharmaceutics. 2020;12(1):45. https://doi.org/10.3390/pharmaceutics12010045.
Kollipara S, Boddu R, Ahmed T, Chachad S. Simplified model-dependent and model-independent approaches for dissolution profile comparison for oral products: regulatory perspective for generic product development. AAPS PharmSciTech. 2022;23(1):1–13. https://doi.org/10.1208/s12249-021-02203-7.
Yoshida H, Shibata H, Izutsu KI, Goda Y. Comparison of dissolution similarity assessment methods for products with large variations: f2 statistics and model-independent multivariate confidence region procedure for dissolution profiles of multiple oral products. Biol Pharm Bull. 2017;40(5):722–5. https://doi.org/10.1248/bpb.b16-00904.
Noce L, Gwaza L, Mangas-Sanjuan V, Garcia-Arieta A. Comparison of free software platforms for the calculation of the 90% confidence interval of f2 similarity factor by bootstrap analysis. Eur J Pharm Sci. 2020;146:105259. https://doi.org/10.1016/j.ejps.2020.105259.
World Health Organization. Proposal to waive in vivo bioequivalence requirements for WHO model list of essential medicines—immediate-release, solid oral dosage forms, Fortieth report, annex 8, WHO, Geneva, Switzerland, 2005. https://www.who.int/medicines/services/expertcommittees/pharmprep/QAS04_109Rev1_Waive_invivo_bioequiv.pdf. Accessed 21 March 2022.
WHO. Who Expert Committee on Specifications for Pharmaceutical Preparations Forty-sixth Report. 2012. WHO Technical Report Series 970. http://www.who.int/medicines/areas/quality_safety/quality_assurance/expert_committee/TRS-970- pdf1.pdf. Accessed 21 March 2022.
Cascone S. Modeling and comparison of release profiles: effect of the dissolution method. Eur J Pharm Sci. 2017;106:352–61. https://doi.org/10.1016/j.ejps.2017.06.021.
Paixão P, Gouveia LF, Silva N, Morais JA. Evaluation of dissolution profile similarity–comparison between the f2, the multivariate statistical distance and the f2 bootstrapping methods. Eur J Pharm Biopharm. 2017;112:67–74. https://doi.org/10.1016/j.ejpb.2016.10.026.
Muselík J, Komersová A, Kubová K, Matzick K, Skalická B. A critical overview of FDA and EMA statistical methods to compare in vitro drug dissolution profiles of pharmaceutical products. Pharmaceutics. 2021;13(10):1703. https://doi.org/10.3390/pharmaceutics13101703.
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, Xie S. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12:263–271. https://doi.org/10.1208/s12248-010-9185-1.
Incecayir T. The effects of surfactants on the solubility and dissolution profiles of a poorly water-soluble basic drug, carvedilol. Pharmazie. 2015;70:784–90. https://doi.org/10.1691/ph.2015.5081.
Wuelfing WP, Kosuda K, Templeton AC, Harman A, Mowery MD, Reed RA. Polysorbate 80 UV/vis spectral and chromatographic characteristics–defining boundary conditions for use of the surfactant in dissolution analysis. J Pharm Biomed Anal. 2006;41(3):774–82. https://doi.org/10.1016/j.jpba.2006.01.020.
Incecayir T, Olgac S, Yılmaz Usta, D, Teksin, ZS. Role of surfactants on dissolution behavior of tamoxifen. Diss Tech. 2021;28(2). https://doi.org/10.14227/DT280221P6.
Polli JE, Rekhi GS, Augsburger LL, Shah VP. Methods to compare dissolution profiles and a rationale for wide dissolution specifications for metoprolol tartrate tablets. J Pharm Sci. 1997;86:690–700. https://doi.org/10.1021/js960473x.
Jain A, RanY, Yalkowsky SH. Effect of pH-sodium lauryl sulfate combination on solubilization of PG-300995 (an anti-HIV agent): a technical note. AAPS PharmSciTech. 2004;5:65–67. https://doi.org/10.1208/pt050345.
Loftsson T, Vogensen SB, Desbos C, Jansook P. Carvedilol: solubilization and cyclodextrin complexation: a technical note. AAPS PharmSciTech. 2008;9:425–30. https://doi.org/10.1208/s12249-008-9055-7.
Tsume Y, Mudie DM, Langguth P, Amidon GE, Amidon GL. The Biopharmaceutics Classification System: subclasses for in vivo predictive dissolution (IPD) methodology and IVIVC. Eur J Pharm Sci. 2014;57:152–63. https://doi.org/10.1016/j.ejps.2014.01.009.
Acknowledgements
The authors would like to thank Drogsan Pharmaceuticals (Ankara, Turkey) for kindly providing the active pharmaceutical ingredient, carvedilol.
Author information
Authors and Affiliations
Contributions
Duygu Yilmaz Usta: Conceptualization, investigation, methodology, writing—review and editing. Tuba Incecayir: Conceptualization, investigation, methodology, formal analysis, supervision, writing—original draft preparation, review, and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Usta, D.Y., Incecayir, T. Modeling of In Vitro Dissolution Profiles of Carvedilol Immediate-Release Tablets in Different Dissolution Media. AAPS PharmSciTech 23, 201 (2022). https://doi.org/10.1208/s12249-022-02355-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02355-0