Abstract
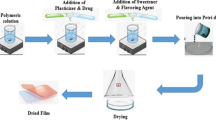
Lysiphyllum strychnifolium has long been used as a popular herbal medicinal plant for treating fever and alcohol intoxication. This study aimed to prepare buccal film for L. strychnifolium stem extracts. These extracts were less soluble in water and were therefore loaded in self-emulsifying systems before being mixed into the film. Astilbin was selected as a chemical marker in L. strychnifolium stem extracts. Firstly, the L. strychnifolium stem extracts were entrapped in the self-emulsifying systems which were designed and optimized based on 32 factorial design. The optimal formulation was 0.60 g of surfactant-co-surfactant mixture (Tween® 80 and polyethylene glycol 400 in the ratio of 7.5:1) and 0.40 g of caprylic/capric triglyceride. Secondly, the optimal self-emulsifying system was loaded in the polymeric film which consisted of polyvinyl alcohol blended with poloxamer 407 using glycerin as a plasticizer. The properties of the prepared buccal film were unchanged, and the film showed an amorphous state, indicating all ingredients might be completely dissolved in the film. The buccal film could be placed in direct contact with the mouth without oral mucosal irritation, and showed a smooth and homogeneous surface with a rough and compact cross-sectional morphology. Astilbin content in the buccal film was 61.39 ± 11.45 µg/cm2. Astilbin was released from the buccal film while the permeation rate was low. The release mechanism was both swelling and diffusion, and followed anomalous or non-Fickian transfer. The permeability coefficient of the cumulative amount of astilbin permeated from buccal film was 1.0192 ± 0.1395 ×10−3 cm/h. Thus, the buccal film can be prepared by using a self-emulsifying system for herbal applications and shows potential as a safe and convenient form of oral drug administration.






Similar content being viewed by others
References
Sukprasert S, Pansuksan K, Sriyakul K. Lysiphyllum strychnifolium (Craib) A Schmitz extract a novel neuraminidase inhibitor of avian influenza virus subtype H5N1. J Herb Med. 2020;20:100330.
Itharat A, Sayompark S, Hansakul P, Dechayont B. In vitro antioxidant activities of extracts of Bauhinia strychnifolia stems and leaves: comparison with activities in green tea extracts. Med Aromat Plants (Los Angel). 2016;5(3):1–7. https://doi.org/10.4172/2167-0412.1000243.
Panchinda C, Ruangnoo S, Itharat A. Cytotoxic activity against cancer cell lines from the ethanolic extracts and its VLC fractions of Bauhinia strychnifolia leaves. J Med Assoc Thai. 2016;99(Suppl 4):S110-5.
Al-Sayed E, Abdel-Daim MM, Kilany OE, Karonen M, Sinkkonen J. Protective role of polyphenols from Bauhinia hookeri against carbon tetrachloride-induced hepato- and nephrotoxicity in mice. Ren Fail. 2015;37(7):1198–207. https://doi.org/10.3109/0886022X.2015.1061886.
Yuenyongsawad S, Bunluepuech K, Wattanapiromsakul C, Tewtrakul S. Anti-cancer activity of compounds from Bauhinia strychnifolia stem. J Ethnopharmacol. 2013;150(2):765–9. https://doi.org/10.1016/j.jep.2013.09.025.
Kaewpiboon C, Lirdprapamongkol K, Srisomsap C, Winayanuwattikun P, Yongvanich T, Puwaprisirisan P, et al. Studies of the in vitro cytotoxic, antioxidant, lipase inhibitory and antimicrobial activities of selected Thai medicinal plants. BMC Complement Altern Med. 2012;12(1):217. https://doi.org/10.1186/1472-6882-12-217.
Sampaopan Y, Kitprapiumpon N, Kongkiatpaiboon S, Duangdee N, Wongyai S. Isolation and HPLC analysis of astilbin in Lysiphyllum strychnifolium (syn Bauhinia strychnifolia) stem. Sci & Tech Asia. 2021;26(1):208–15.
Zhou Q, Lu W, Niu Y, Liu J, Zhang X, Gao B, et al. Identification and quantification of phytochemical composition and anti-inflammatory, cellular antioxidant, and radical scavenging activities of 12 Plantago species. J Agric Food Chem. 2013;61(27):6693–702. https://doi.org/10.1021/jf401191q.
Cai Y, Chen T, Xu Q. Astilbin suppresses collagen-induced arthritis via the dysfunction of lymphocytes. Inflamm Res. 2003;52(8):334–40. https://doi.org/10.1007/s00011-003-1179-3.
Wang J, Zhao Y, Xu Q. Astilbin prevents concanavalin A-induced liver injury by reducing TNF-α production and T lymphocyte adhesion. J Pharm Pharmacol. 2004;56(4):495–502. https://doi.org/10.1211/0022357023033.
Fuhrmann K, Fuhrmann G. Recent advances in oral delivery of macromolecular drugs and benefits of polymer conjugation. Curr Opin Colloid Interface Sci. 2017;31:67–74. https://doi.org/10.1016/j.cocis.2017.07.002.
Wannaphatchaiyong S, Heng PWS, Suksaeree J, Boonme P, Pichayakorn W. Lidocaine loaded gelatin/gelatinized tapioca starch films for buccal delivery and the irritancy evaluation using chick chorioallantoic membrane. Saudi Pharm J. 2019;27(8):1085–95. https://doi.org/10.1016/j.jsps.2019.09.005.
Xiao L, Yi T, Liu Y. A new self-microemulsifying mouth dissolving film to improve the oral bioavailability of poorly water soluble drugs. Drug Dev Ind Pharm. 2013;39(9):1284–90. https://doi.org/10.3109/03639045.2012.723716.
Hassan Mahboob MB, Riaz T, Jamshaid M, Bashir I, Zulfiqar S. Oral films: a comprehensive review. Int Curr Pharm J. 2016;5(12):111–7. https://doi.org/10.3329/icpj.v5i12.30413.
Montenegro-Nicolini M, Morales JO. Overview and future potential of buccal mucoadhesive films as drug delivery systems for biologics. AAPS PharmSciTech. 2017;18(1):3–14. https://doi.org/10.1208/s12249-016-0525-z.
Alaei S, Omidian H. Mucoadhesion and mechanical assessment of oral films. Eur J Pharm Sci. 2021;159:105727. https://doi.org/10.1016/j.ejps.2021.105727.
Suksaeree J, Chaichawawut B, Srichan M, Tanaboonsuthi N, Monton C, Maneewattanapinyo P, et al. Applying design of experiments (DoE) on the properties of buccal film for nicotine delivery. E-Polym. 2021;21(1):566–74.
Krull SM, Ma Z, Li M, Davé RN, Bilgili E. Preparation and characterization of fast dissolving pullulan films containing BCS class II drug nanoparticles for bioavailability enhancement. Drug Dev Ind Pharm. 2016;42(7):1073–85. https://doi.org/10.3109/03639045.2015.1107094.
Bala R, Sharma S. Formulation optimization and evaluation of fast dissolving film of aprepitant by using design of experiment. Bull Fac Pharm Cairo Univ. 2018;56(2):159–68. https://doi.org/10.1016/j.bfopcu.2018.04.002.
Bala R, Madaan R, Gupta R, Chawla R, Sahoo U. Formulation of mouth dissolving strips of metoprolol succinate using locust bean gum. Egypt J Chem. 2021;64(1):187–92. https://doi.org/10.21608/ejchem.2020.38329.2789.
Mostafavi FS, Kadkhodaee R, Emadzadeh B, Koocheki A. Preparation and characterization of tragacanth–locust bean gum edible blend films. Carbohydr Polym. 2016;139:20–7. https://doi.org/10.1016/j.carbpol.2015.11.069.
Lai F, Franceschini I, Corrias F, Sala MC, Cilurzo F, Sinico C, et al. Maltodextrin fast dissolving films for quercetin nanocrystal delivery. A feasibility study Carbohydr Polym. 2015;121:217–23. https://doi.org/10.1016/j.carbpol.2014.11.070.
Al-Mogherah AI, Ibrahim MA, Hassan MA. Optimization and evaluation of venlafaxine hydrochloride fast dissolving oral films. Saudi Pharm J. 2020;28(11):1374–82. https://doi.org/10.1016/j.jsps.2020.09.001.
Kevadiya BD, Barvaliya M, Zhang L, Anovadiya A, Brahmbhatt H, Paul P, et al. Fenofibrate nanocrystals embedded in oral strip-films for bioavailability enhancement. Bioengineering. 2018;5(1):16.
Prabhu P, Malli R, Koland M, Vijaynarayana K, D’Souza U, Harish N, et al. Formulation and evaluation of fast dissolving films of levocitirizine di hydrochloride. Int J Pharm Investig. 2011;1(2):99–104. https://doi.org/10.4103/2230-973X.82417.
Pattewar SV, Kasture SB, Pande VV, Sharma SK. A new self microemulsifying mouth dissolving film. Indian J Pharm Educ Res. 2016;50(3s):S191–9. https://doi.org/10.5530/ijper.50.3.29.
Chairuk P, Tubtimsri S, Jansakul C, Sriamornsak P, Weerapol Y. Enhancing oral absorption of poorly water-soluble herb (Kaempferia parviflora) extract using self-nanoemulsifying formulation. Pharm Dev Technol. 2020;25(3):340–50. https://doi.org/10.1080/10837450.2019.1703134.
Mandić J, Zvonar Pobirk A, Vrečer F, Gašperlin M. Overview of solidification techniques for self-emulsifying drug delivery systems from industrial perspective. Int J Pharm. 2017;533(2):335–45. https://doi.org/10.1016/j.ijpharm.2017.05.036.
Rani S, Rana R, Saraogi GK, Kumar V, Gupta U. Self-emulsifying oral lipid drug delivery systems: advances and challenges. AAPS PharmSciTech. 2019;20(3):129. https://doi.org/10.1208/s12249-019-1335-x.
Acharya SP, Pundarikakshudu K, Panchal A, Lalwani A. Preparation and evaluation of transnasal microemulsion of carbamazepine. Asian J Pharm Sci. 2013;8(1):64–70. https://doi.org/10.1016/j.ajps.2013.07.008.
Ramli S, Chyi KT, Zainuddin N, Mokhtar WNAW, Abdul Rahman I. The influence of surfactant/co-surfactant hydrophilic-lipophilic balance on the formation of limonene-based microemulsion as vitamin C carrier. Sains Malays. 2019;48(5):1035–42.
Fea P, Nur AA, Marti H, Tetri W, Zuhra NS, Rengganis W. Formulating self-microemulsifying drug delivery systems from bay leaves (Eugenia polyantha Wight) with virgin coconut oil and its antidiabetic activity. Iran J Pharm Sci. 2020;16(2):45–56.
Salam Shanta T, Khalid Kadhem AK, Zahraa Mohsen H, Mowafaq mohammed G. Co-surfactant effect of polyethylene glycol 400 on microemulsion using BCS class II model drug. J Adv Pharm Educ Res. 2022;12(1):63–9. https://doi.org/10.51847/1h17TZqgyI.
Mungali M, Sharma N, Gauri. Caprylic/capric triglyceride. In: Belwal T, Nabavi SM, Nabavi SF, Dehpour AR, Shirooie S, editors. Naturally occurring chemicals against Alzheimer’s disease: Academic Press; 2021 139-46.
Sampaopan Y, Suksaeree J. Formulation development and pharmaceutical evaluation of Lysiphyllum strychnifolium topical patches for their anti-inflammatory potential. AAPS PharmSciTech. 2022;23(5):116. https://doi.org/10.1208/s12249-022-02269-x.
Sharma A, Gupta S, Chauhan S, Nair A, Sharma P. Astilbin: a promising unexplored compound with multidimensional medicinal and health benefits. Pharmacol Res. 2020;158:104894. https://doi.org/10.1016/j.phrs.2020.104894
Yu L, Huang H, Yu L, Wang TTY. Utility of hesperidinase for food function research: enzymatic digestion of botanical extracts alters cellular antioxidant capacities and anti-inflammatory properties. J Agric Food Chem. 2014;62(34):8640–7. https://doi.org/10.1021/jf501963a.
Marques MRC, Loebenberg R, Almukainzi M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011:15-28. https://doi.org/10.14227/DT180311P15.
Czajkowska-Kośnik A, Szekalska M, Amelian A, Szymańska E, Winnicka K. Development and evaluation of liquid and solid self-emulsifying drug delivery systems for atorvastatin. Molecules. 2015;20(12):21010–22.
Tejada G, Barrera MG, Piccirilli GN, Sortino M, Frattini A, Salomón CJ, et al. Development and evaluation of buccal films based on chitosan for the potential treatment of oral candidiasis. AAPS PharmSciTech. 2017;18(4):936–46. https://doi.org/10.1208/s12249-017-0720-6.
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, et al. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12(3):263–71. https://doi.org/10.1208/s12248-010-9185-1
Chuadee S, Kitsongsermthon J. Effect of polymers and glycerin on buccal films containing asiaticoside and aloe vera extract. Thai J Pharm Sci. 2017;41:209–12.
Suksaeree J, Waiprib R, Pichayakorn W. Improving the hydrophilic properties of deproteinized natural rubber latex films for lidocaine transdermal patches by starch blending. J Polym Environ. 2022;30(4):1574–86. https://doi.org/10.1007/s10924-021-02285-1.
Suksaeree J, Waiprib R, Kalkornsurapranee E, Pichayakorn W. Lidocaine-pressure sensitive adhesive patches from STR-5L block rubber: preparations, in vitro characterizations, and stability studies. J Drug Deliv Sci Technol. 2022;67:102966. https://doi.org/10.1016/j.jddst.2021.102966.
Abouhussein D, El Nabarawi MA, Shalaby SH, El-Bary AA. Cetylpyridinium chloride chitosan blended mucoadhesive buccal films for treatment of pediatric oral diseases. J Drug Deliv Sci Technol. 2020;57:101676. https://doi.org/10.1016/j.jddst.2020.101676.
Alsofany JM, Hamza MY, Abdelbary AA. Fabrication of nanosuspension directly loaded fast-dissolving films for enhanced oral bioavailability of olmesartan medoxomil: in vitro characterization and pharmacokinetic evaluation in healthy human volunteers. AAPS PharmSciTech. 2018;19(5):2118–32. https://doi.org/10.1208/s12249-018-1015-2.
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57. https://doi.org/10.3390/pharmaceutics10020057.
Lupo N, Steinbring C, Friedl JD, Le-Vinh B, Bernkop-Schnürch A. Impact of bile salts and a medium chain fatty acid on the physical properties of self-emulsifying drug delivery systems. Drug Dev Ind Pharm. 2021;47(1):22–35. https://doi.org/10.1080/03639045.2020.1851241.
Suhaimi SH, Hasham Hisam R, Rosli NA. Effects of formulation parameters on particle size and polydispersity index of Orthosiphon stamineus loaded nanostructured lipid carrier. Int J Adv Res Sci Eng Technol. 2020;1(1):36–9.
Vasconcelos T, Marques S, Sarmento B. Measuring the emulsification dynamics and stability of self-emulsifying drug delivery systems. Eur J Pharm Biopharm. 2018;123:1–8. https://doi.org/10.1016/j.ejpb.2017.11.003.
Kumar A, Dixit CK. Methods for characterization of nanoparticles. In: Nimesh S, Chandra R, Gupta N, editors. Advances in nanomedicine for the delivery of therapeutic nucleic acids: Woodhead Publishing; 2017. 43-58.
Seng LY, Al-Shaikh M, Hascakir B. Intermolecular interaction between heavy crude oils and surfactants during surfactant-steam flooding process. ACS Omega. 2020;5(42):27383–92. https://doi.org/10.1021/acsomega.0c00193.
Bernkop-Schnürch A, Jalil A. Do drug release studies from SEDDS make any sense? J Contr Release. 2018;271:55–9. https://doi.org/10.1016/j.jconrel.2017.12.027.
Agarwal V, Siddiqui A, Ali H, Nazzal S. Dissolution and powder flow characterization of solid self-emulsified drug delivery system (SEDDS). Int J Pharm. 2009;366(1):44–52. https://doi.org/10.1016/j.ijpharm.2008.08.046.
Milović M, Djuriš J, Djekić L, Vasiljević D, Ibrić S. Characterization and evaluation of solid self-microemulsifying drug delivery systems with porous carriers as systems for improved carbamazepine release. Int J Pharm. 2012;436(1):58–65. https://doi.org/10.1016/j.ijpharm.2012.06.032.
Asgarirad H, Ebrahimnejad P, Mahjoub MA, Jalalian M, Morad H, Ataee R, et al. A promising technology for wound healing; in-vitro and in-vivo evaluation of chitosan nano-biocomposite films containing gentamicin. J Microencapsul. 2021;38(2):100–7. https://doi.org/10.1080/02652048.2020.1851789.
Al-Nemrawi NK, Alsharif SSM, Alzoubi KH, Alkhatib RQ. Preparation and characterization of insulin chitosan-nanoparticles loaded in buccal films. Pharm Dev Technol. 2019;24(8):967–74. https://doi.org/10.1080/10837450.2019.1619183.
Suksaeree J, Monton C, Madaka F, Chusut T, Saingam W, Pichayakorn W, et al. Formulation, physicochemical characterization, and in vitro study of chitosan/HPMC blends-based herbal blended patches. AAPS PharmSciTech. 2015;16(1):171–81. https://doi.org/10.1208/s12249-014-0216-6.
Monton C, Sampaopan Y, Pichayakorn W, Panrat K, Suksaeree J. Herbal transdermal patches made from optimized polyvinyl alcohol blended film: herbal extraction process, film properties, and in vitro study. J Drug Deliv Sci Technol. 2022;69:103170. https://doi.org/10.1016/j.jddst.2022.103170.
Muzib YI, Kumari KS. Mucoadhesive buccal films of glibenclamide: development and evaluation. Int J Pharm Investig. 2011;1(1):42–7. https://doi.org/10.4103/2230-973x.76728.
Zhang S, Kim N, Yokoyama W, Kim Y. Effects of moisture content on mechanical properties, transparency, and thermal stability of yuba film. Food Chem. 2018;243:202–7. https://doi.org/10.1016/j.foodchem.2017.09.127.
Suksaeree J, Karnsopa P, Wannaphruek N, Prasomkij J, Panrat K, Pichayakorn W. Transdermal delivery of nicotine using pectin isolated from durian fruit-hulls-based polymer blends as a matrix layer. J Polym Environ. 2018;26(8):3216–25. https://doi.org/10.1007/s10924-018-1203-x.
Mahendia S, Heena, Kandhol G, Deshpande UP, Kumar S. Determination of glass transition temperature of reduced graphene oxide-poly(vinyl alcohol) composites using temperature dependent Fourier transform infrared spectroscopy. J Mol Struct. 2016;1111:46–54. https://doi.org/10.1016/j.molstruc.2016.01.072
Patel AK, Bajpai R, Keller JM. On the crystallinity of PVA/palm leaf biocomposite using DSC and XRD techniques. Microsyst Technol. 2014;20(1):41–9. https://doi.org/10.1007/s00542-013-1882-0.
Guan Y, Shao L, Dong D, Wang F, Zhang Y, Wang Y. Bio-inspired natural polyphenol cross-linking poly(vinyl alcohol) films with strong integrated strength and toughness. RSC Adv. 2016;6(74):69966–72. https://doi.org/10.1039/C6RA08904F.
Peng M, Xiao G, Tang X, Zhou Y. Hydrogen-bonding assembly of rigid-rod poly(p-sulfophenylene terephthalamide) and flexible-chain poly(vinyl alcohol) for transparent, strong, and tough molecular composites. Macromolecules. 2014;47(23):8411–9. https://doi.org/10.1021/ma501590x.
Caon T, Jin L, Simões CMO, Norton RS, Nicolazzo JA. Enhancing the buccal mucosal delivery of peptide and protein therapeutics. Pharm Res. 2015;32(1):1–21. https://doi.org/10.1007/s11095-014-1485-1.
Hanif M, Zaman M. Thiolation of arabinoxylan and its application in the fabrication of controlled release mucoadhesive oral films. DARU J Pharm Sci. 2017;25(1):6. https://doi.org/10.1186/s40199-017-0172-2.
Acknowledgements
The authors would like to thank the following research assistants: Wannaput Nitiakkarapong, Natchanon Jiramongkolrath, Jiraphat Wingwon, Kietwinee Buaphet, Jessada Prasomkij, and Chanchai Suwanlaong. This article was proofread and edited by Cambridge Proofreading LLC, which was funded by Rangsit University’s Research Institute.
Funding
This research was financially supported by the College of Pharmacy, Rangsit University.
Author information
Authors and Affiliations
Contributions
Wiwat Pichayakorn and Chaowalit Monton: research, paper conception, data processing, data analysis, preparation of figures, discussion of results, and paper writing. Yupaporn Sampaopan and Kamon Panrat: data processing, data analysis, and discussion of results. Jirapornchai Suksaeree: advisor professor, research, paper conception, data processing, data analysis, preparation of graphs and figures, literature review, discussion of results, paper writing, and critical revision and approval final of article.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pichayakorn, W., Monton, C., Sampaopan, Y. et al. Fabrication and Characterization of Buccal Film Loaded Self-emulsifying Drug Delivery System containing Lysiphyllum strychnifolium Stem Extracts. AAPS PharmSciTech 23, 194 (2022). https://doi.org/10.1208/s12249-022-02341-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02341-6