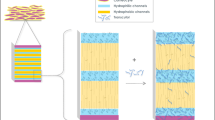
Abstract
Physicochemical and formulation factors influencing penetration of drugs from topical products into the skin and mechanisms of drug permeation are well investigated and reported in the literature. However, mechanisms of drug absorption during short-term exposure have not been given sufficient importance. In this project, the extent of absorption of drug molecules into the skin from aqueous and ethanolic solutions following a 5-min application period was investigated. The experiments demonstrated measurable magnitude of absorption into the skin for all the molecules tested despite the duration of exposure being only few minutes. Among the two solvents used, absorption was greater from aqueous than ethanolic solution. The results suggest that an alternative penetration pathway, herein referred to as the convective transport pathway, is likely responsible for the rapid, significant uptake of drug molecules during initial few minutes of exposure. Additionally, absorption through the convective transport pathways is a function of the physicochemical nature of the formulation vehicle rather than the API.




Similar content being viewed by others
References
Naik A, Kalia YN, Guy RHJPs, today t. Transdermal drug delivery: overcoming the skin’s barrier function. 2000;3(9):318–26.
Bommannan D, Potts RO, Guy RH. Examination of stratum corneum barrier function in vivo by infrared spectroscopy. J Investig Dermatol. 1990;95(4):403–8.
Fitzpatrick D, Corish J, Hayes BJC. Modelling skin permeability in risk assessment––the future. 2004;55(10):1309–14.
Godin B, Touitou EJAddr. Transdermal skin delivery: predictions for humans from in vivo, ex vivo and animal models. 2007;59(11):1152-61.
Wilschut A, Wil F, Robinson PJ, McKone TEJC. Estimating skin permeation. The validation of five mathematical skin permeation models. 1995;30(7):1275–96.
Potts RO, Guy RHJPr. Predicting skin permeability. 1992;9(5):663–9.
Guy R, Maibach H. Rapid radial transport of methyl nicotinate in the dermis. Arch Dermatol Res. 1982;273(1–2):91–5.
Guy R, Tur E, Schall L, Elamir S, Maibach H. Determination of vehicle effects on percutaneous absorption by laser Doppler velocimetry. Arch Dermatol Res. 1986;278(6):500–2.
Wester RC, Hui X, Maibach HI. In vivo human transfer of topical bioactive drug between individuals: estradiol. J Investig Dermatol. 2006;126(10):2190–3.
Woolf A, Burkhart K, Caraccio T, Litovitz T. Childhood poisoning involving transdermal nicotine patches. Pediatrics. 1997;99(5):e4-e.
Gardner-Nix J. Caregiver toxicity from transdermal fentanyl. J Pain Symptom Manage. 2001;21(6):447–8.
Valdez CA, Leif RN, Mayer BPJPO. An efficient, optimized synthesis of fentanyl and related analogs. 2014;9(9):e108250.
Davies DJ, Ward R, Heylings J. Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicol In Vitro. 2004;18(3):351–8.
Wilhelm K-P, Cua AB, Wolff HH, Maibach HI. Surfactant-induced stratum corneum hydration in vivo: prediction of the irritation potential of anionic surfactants. J Investig Dermatol. 1993;101(3):310–5.
Van der Molen R, Spies Ft, Van‘t Noordende J, Boelsma E, Mommaas A, Koerten H. Tape stripping of human stratum corneum yields cell layers that originate from various depths because of furrows in the skin. Archives of dermatological research. 1997;289(9):514–8.
Jacobi U, Weigmann HJ, Ulrich J, Sterry W, Lademann J. Estimation of the relative stratum corneum amount removed by tape stripping. Skin Research and Technology. 2005;11(2):91–6.
Huynh N-H, Tyrefors N, Ekman L, Johansson MJJop, analysis b. Determination of fentanyl in human plasma and fentanyl and norfentanyl in human urine using LC–MS/MS. 2005;37(5):1095–100.
Narasimha Murthy S, Wiskirchen DE, Paul BC. Iontophoretic drug delivery across human nail. J Pharm Sci. 2007;96(2):305–11.
Maurya A, Murthy SNJJops. Pretreatment with skin permeability enhancers: importance of duration and composition on the delivery of diclofenac sodium. 2014;103(5):1497–503.
Prausnitz MR, Elias PM, Franz TJ, Schmuth M, Tsai J-C, Menon GK, et al. Skin barrier and transdermal drug delivery. Dermatology. 2012;3:2065–73.
Ng KW, Lau WM. Skin deep: the basics of human skin structure and drug penetration. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Springer; 2015. p. 3–11.
Scheuplein RJ. Mechanism of percutaneous absorption: II Transient diffusion and the relative importance of various routes of skin penetration. Journal of Investigative Dermatology. 1967;48(1):79–88.
Cleek RL, Bunge ALJPR. A new method for estimating dermal absorption from chemical exposure 1. General approach. 1993;10(4):497–506.
Bunge AL, Cleek RL, Vecchia BEJPr. A new method for estimating dermal absorption from chemical exposure. 3. Compared with steady-state methods for prediction and data analysis. 1995;12(7):972–82.
Bogen KT, Keating GA, Meissner S, Vogel JSJJoea, epidemiology e. Initial uptake kinetics in human skin exposed to dilute aqueous trichloroethylene in vitro. 1998;8(2):253–71.
Heisig M, Lieckfeldt R, Wittum G, Mazurkevich G, Lee GJPr. Non steady-state descriptions of drug permeation through stratum corneum. I. The biphasic brick-and-mortar model. 1996;13(3):421–6.
McDougal JN, Jurgens‐Whitehead JLJRA. Short‐term dermal absorption and penetration of chemicals from aqueous solutions: theory and experiment. 2001;21(4):719–26.
Bogen KT, Keating GAJIP. Dermal absorption from short-term exposure to contaminated water. 2000;(260):101–10.
Jacobi U, Kaiser M, Toll R, Mangelsdorf S, Audring H, Otberg N, et al. Porcine ear skin: an in vitro model for human skin. 2007;13(1):19–24.
Jacobi U, Weigmann HJ, Ulrich J, Sterry W, Lademann JJSR, Technology. Estimation of the relative stratum corneum amount removed by tape stripping. 2005;11(2):91–6.
Olesen CM, Fuchs CSK, Philipsen PA, Hædersdal M, Agner T, Clausen M-LJSr. Advancement through epidermis using tape stripping technique and reflectance confocal microscopy. 2019;9(1):1–6.
Potts RO, Guy RH. Predicting skin permeability. Pharm Res. 1992;9(5):663–9.
Murakami T, Ihara C, Kiyonaka G, Yumoto R, Shigeki S, Ikuta Y, et al. Iontophoretic transdermal delivery of salicylic acid dissolved in ethanol-water mixture in rats. Skin Pharmacology and Physiology. 1999;12(4):221–6.
Veryser L, Boonen J, Mehuys E, Roche N, Remon J-P, Peremans K, et al. Transdermal evaluation of caffeine in different formulations and excipients. Journal of Caffeine Research. 2013;3(1):41–6.
Moore C, Wilkinson S, Blain P, Dunn M, Aust G, Williams FJTl. Percutaneous absorption and distribution of organophosphates (chlorpyrifos and dichlorvos) following dermal exposure and decontamination scenarios using in vitro human skin model. 2014;229(1):66–72.
Smith HW, CLOWES GH, Marshall E. On dichloroethylsulfide (mustard gas) IV. The mechanism of absorption by the skin. Journal of Pharmacology and Experimental Therapeutics. 1919;13(1):1–30.
Delanoe D, Fougeyrollas B, Meyer L, Thonneau P. Androgenisation of female partners of men on medroxyprogesterone acetate/percutaneous testosterone contraception. The Lancet. 1984;323(8371):276.
Sakdiset P, Kitao Y, Todo H, Sugibayashi K. High-throughput screening of potential skin penetration-enhancers using stratum corneum lipid Liposomes: preliminary evaluation for different concentrations of ethanol. Journal of pharmaceutics. 2017;2017.
Babu RJ, Pandit JK. Effect of penetration enhancers on the transdermal delivery of bupranolol through rat skin. Drug Delivery. 2005;12(3):165–9.
Femenia-Font A, Balaguer-Fernandez C, Merino V, Rodilla V, Lopez-Castellano A. Effect of chemical enhancers on the in vitro percutaneous absorption of sumatriptan succinate. Eur J Pharm Biopharm. 2005;61(1–2):50–5.
Liu X, Quan P, Li S, Liu C, Zhao Y, Zhao Y, et al. Time dependence of the enhancement effect of chemical enhancers: molecular mechanisms of enhancing kinetics. J Control Release. 2017;248:33–44.
Dupuis D, Rougier A, Roguet R, LOTTE C. The measurement of the stratum corneum reservoir: a simple method to predict the influence of vehicles on in vivo percutaneous absorption. British Journal of Dermatology. 1986;115(2):233–8.
Franklin SL, Geffner ME. Precocious puberty secondary to topical testosterone exposure. J Pediatr Endocrinol Metab. 2003;16(1):107–10.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design: Abhijeet Maurya, S. Narasimha Murthy, and Jungeun Bae. Analysis and interpretation of results: S Narasimha Murthy and Howard Maibach. Draft manuscript preparation: Abhijeet Maurya, S. Narasimha Murthy, H.N. Shivakumar, and Vanaja K. All authors read, approved, and actively participated in revising the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Guest Edited by Jayachandra Babu Ramapuram and Ashana Puri.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maurya, A., Bae, J., Kenchappa, V. et al. Convective Solvent Transport Pathways for Absorption of Drugs from Topical Formulation. AAPS PharmSciTech 23, 178 (2022). https://doi.org/10.1208/s12249-022-02320-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02320-x