Abstract
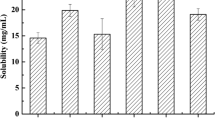
Diosmetin (DIOS) is a functional compound with poor water solubility, bad permeability, and crystal form. Self-microemulsifying drug delivery system (SMEDDS) was an effective formulation to overcome these shortcomings. In this study, liquid SMEDDS was prepared using Capmul® MCM C8 EP/NF, Cremophor EL, and PEG 400 (2:5.6:2.4, w/w/w) as excipients. Then, the novel technology of electrospray solidified liquid SMEDDS and prepared solid SMEDDS for inhibiting crystallization. Polyvinyl pyrrolidone (PVP) was used as carrier to construct DIOS-loaded solid SMEDDS, with polyethylene oxide (PEO) contributing to the formation of regular sphere in the process of spinning. The particle size of solid SMEDDS (194 ± 5 nm) was much bigger than of liquid SMEDDS (25 ± 1 nm), while DIOS-loaded solid SMEDDS showed greater dissolution rates in pH 1.2 and pH 6.8 media through in vitro drug release study. The solid nanoparticles were smooth and uniform from the graph of a scanning electron microscope (SEM). The graph of a transmission electron microscope (TEM) showed that small droplets were loaded in the matrix. Furthermore, DIOS was encapsulated by matrix in amorphous state via differential scanning calorimetry (DSC) and attenuated total reflection Fourier transform infrared (ATR-FTIR). The crystalline of DIOS was not formed in solid SMEDDS due to the characteristic peaks of DIOS disappeared in X-ray diffraction (XRD) pattern. Therefore, the oral bioavailability of DIOS improved significantly compared with liquid SMEDDS (4.27-fold). Hence, solid SMEDDS could improve the solubility and bioavailability of DIOS, through transfer of the state of crystalline to amorphous by electrospray technology.








Similar content being viewed by others
References
Fang X, Gao W, Yang Z, Gao Z, Li H. Dual anti-/prooxidant behaviors of flavonoids pertaining to Cu(II)-catalyzed tyrosine nitration of the insulin receptor kinase domain in an antidiabetic study. J Agric Food Chem. 2020;68(22):6202–11. https://doi.org/10.1021/acs.jafc.0c01676.
Bajraktari G, Weiss J. The aglycone diosmetin has the higher perpetrator drug-drug interaction potential compared to the parent flavone diosmin. J Funct Foods. 2020;67:103842. https://doi.org/10.1016/j.jff.2020.103842.
Ma A, Zhang R. Diosmetin inhibits cell proliferation, induces cell apoptosis and cell cycle arrest in liver cancer. Cancer Manag Res. 2020;12:3537–46. https://doi.org/10.2147/CMAR.S240064.
Chen J-J, Zhang J-X, Zhang X-Q, Qi M-J, Shi M-Z, Yang J, et al. Effects of diosmetin on nine cytochrome P450 isoforms, UGTs and three drug transporters in vitro. Toxicol Appl Pharmacol. 2017;334:1–7. https://doi.org/10.1016/j.taap.2017.08.020.
Zaragoza C, Villaescusa L, Monserrat J, Zaragoza F, Alvarez-Mon M. Potential therapeutic anti-inflammatory and immunomodulatory effects of dihydroflavones, flavones, and flavonols. Molecules. 2020;25(4):1017. https://doi.org/10.3390/molecules25041017.
Li F, Hu R, Wang B, Gui Y, Cheng G, Gao S, et al. Self-microemulsifying drug delivery system for improving the bioavailability of huperzine A by lymphatic uptake. Acta Pharm Sin B. 2017;7(3):353–60. https://doi.org/10.1016/j.apsb.2017.02.002.
Zhu Z, Liu J, Yang Y, Adu-Frimpong M, Ji H, Toreniyazov E, et al. SMEDDS for improved oral bioavailability and anti-hyperuricemic activity of licochalcone A. J Microencapsul. 2021:1–13. https://doi.org/10.1080/02652048.2021.1963341.
Huo T, Tao C, Zhang M, Liu Q, Lin B, Liu Z, et al. Preparation and comparison of tacrolimus-loaded solid dispersion and self-microemulsifying drug delivery system by in vitro/in vivo evaluation. Eur J Pharm Sci. 2018;114:74–83. https://doi.org/10.1016/j.ejps.2017.12.002.
Cao X, Zhu Q, Wang QL, Adu-Frimpong M, Wei CM, Weng W, et al. Improvement of oral bioavailability and anti-tumor effect of zingerone self-microemulsion drug delivery system. J Pharm Sci. 2021;110(7):2718–27. https://doi.org/10.1016/j.xphs.2021.01.037.
Vithani K, Hawley A, Jannin V, Pouton C, Boyd BJ. Solubilisation behaviour of poorly water-soluble drugs during digestion of solid SMEDDS. Eur J Pharm Biopharm. 2018;130:236–46. https://doi.org/10.1016/j.ejpb.2018.07.006.
Guan J, Huan X, Liu Q, Jin L, Wu H, Zhang X, et al. Synergetic effect of nucleation and crystal growth inhibitor on in vitro-in vivo performance of supersaturable lacidipine solid dispersion. Int J Pharm. 2019;566:594–603. https://doi.org/10.1016/j.ijpharm.2019.06.010.
Oh DH, Kang JH, Kim DW, Lee BJ, Kim JO, Yong CS, et al. Comparison of solid self-microemulsifying drug delivery system (solid SMEDDS) prepared with hydrophilic and hydrophobic solid carrier. Int J Pharm. 2011;420(2):412–8. https://doi.org/10.1016/j.ijpharm.2011.09.007.
Elik A, Koçak Yanık D, Göğüş F. A comparative study of encapsulation of carotenoid enriched-flaxseed oil and flaxseed oil by spray freeze-drying and spray drying techniques. LWT. 2021;143:111153. https://doi.org/10.1016/j.lwt.2021.111153.
Tanhaei A, Mohammadi M, Hamishehkar H, Hamblin MR. Electrospraying as a novel method of particle engineering for drug delivery vehicles. J Control Release. 2020. https://doi.org/10.1016/j.jconrel.2020.10.059.
Wang P, Li Y, Jiang M. Effects of the multilayer structures on Exenatide release and bioactivity in microsphere/thermosensitive hydrogel system. Colloids Surf B Biointerfaces. 2018;171:85–93. https://doi.org/10.1016/j.colsurfb.2018.04.063.
Karimi A, Askari G, Yarmand MS, Salami M, EmamDjomeh Z. Development, modification and characterization of ursolic acid-loaded gelatin nanoparticles through electrospraying technique. Food Bioprod Process. 2020;124:329–41. https://doi.org/10.1016/j.fbp.2020.08.018.
Al-Shdefat R, Anwer MK, Fayed MH, Alsulays BB, Tawfeek HM, Abdel-Rahman RF, et al. Preparation and evaluation of spray dried rosuvastatin calcium-PVP microparticles for the improvement of serum lipid profile. J Drug Deliv Sci Technol. 2020;55:101342. https://doi.org/10.1016/j.jddst.2019.101342.
Menazea AA. One-pot pulsed laser ablation route assisted copper oxide nanoparticles doped in PEO/PVP blend for the electrical conductivity enhancement. J Market Res. 2020;9(2):2412–22. https://doi.org/10.1016/j.jmrt.2019.12.073.
Abdelrazek EM, Abdelghany AM, Badr SI, Morsi MA. Structural, optical, morphological and thermal properties of PEO/PVP blend containing different concentrations of biosynthesized Au nanoparticles. J Market Res. 2018;7(4):419–31. https://doi.org/10.1016/j.jmrt.2017.06.009.
Dhatarwal P, Sengwa RJ, Choudhary S. Multifunctional (PVP/PEO)/SnO2 nanocomposites of tunable optical and dielectric properties. Optik. 2020;221:165368. https://doi.org/10.1016/j.ijleo.2020.165368.
Bharate SS. Enhancing biopharmaceutical attributes of khellin by amorphous binary solid dispersions. AAPS PharmSciTech. 2021;22(8):260. https://doi.org/10.1208/s12249-021-02126-3.
Sammani MS, Clavijo S, Portugal L, Suarez R, Seddik H, Cerda V. Use of multiresponse statistical techniques to optimize the separation of diosmin, hesperidin, diosmetin and hesperitin in different pharmaceutical preparations by high performance liquid chromatography with UV-DAD. Talanta. 2017;167:695–702. https://doi.org/10.1016/j.talanta.2017.02.069.
Zhu Y, Xu W, Zhang J, Liao Y, Firempong CK, Adu-Frimpong M, et al. Self-microemulsifying drug delivery system for improved oral delivery of limonene: preparation, characterization, in vitro and in vivo evaluation. AAPS PharmSciTech. 2019;20(4):153. https://doi.org/10.1208/s12249-019-1361-8.
Wang L, Yan W, Tian Y, Xue H, Tang J, Zhang L. Self-microemulsifying drug delivery system of phillygenin: formulation development, characterization and pharmacokinetic evaluation. Pharmaceutics. 2020;12(2):130. https://doi.org/10.3390/pharmaceutics12020130.
Parveen R, Baboota S, Ali J, Ahuja A, Vasudev SS, Ahmad S. Oil based nanocarrier for improved oral delivery of silymarin: in vitro and in vivo studies. Int J Pharm. 2011;413(1–2):245–53. https://doi.org/10.1016/j.ijpharm.2011.04.041.
Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 2007;66(2):227–43. https://doi.org/10.1016/j.ejpb.2006.10.014.
Soleimanifar M, Jafari SM, Assadpour E. Encapsulation of olive leaf phenolics within electrosprayed whey protein nanoparticles; production and characterization. Food Hydrocolloids. 2020;101:105572. https://doi.org/10.1016/j.foodhyd.2019.105572.
Bottani M, Cornaghi L, Donetti E, Ferraretto A. Excess of nutrient-induced morphofunctional adaptation and inflammation degree in a Caco2/HT-29 in vitro intestinal co-culture. Nutrition. 2019;58:156–66. https://doi.org/10.1016/j.nut.2018.07.018.
Sun C, Li W, Zhang H, Adu-Frimpong M, Ma P, Zhu Y, et al. Improved oral bioavailability and hypolipidemic effect of syringic acid via a self-microemulsifying drug delivery system. AAPS PharmSciTech. 2021;22(1):45. https://doi.org/10.1208/s12249-020-01901-y.
Shen Q, Li X, Yuan D, Jia W. Enhanced oral bioavailability of daidzein by self-microemulsifying drug delivery system. Chem Pharm Bull (Tokyo). 2010;58(5):639–43. https://doi.org/10.1248/cpb.58.639.
Jouyandeh M, Ganjali MR, Ali JA, Aghazadeh M, Saeb MR, Ray SS. Curing epoxy with polyvinylpyrrolidone (PVP) surface-functionalized NixFe3-xO4 magnetic nanoparticles. Prog Org Coat. 2019;136:105259. https://doi.org/10.1016/j.porgcoat.2019.105259.
Doreth M, Löbmann K, Priemel P, Grohganz H, Taylor R, Holm R, et al. Influence of PVP molecular weight on the microwave assisted in situ amorphization of indomethacin. Eur J Pharm Biopharm. 2018;122:62–9. https://doi.org/10.1016/j.ejpb.2017.10.001.
Aswar M, Bhalekar M, Trimukhe A, Aswar U. Self-microemulsifying drug delivery system (SMEDDS) of curcumin attenuates depression in olfactory bulbectomized rats. Heliyon. 2020;6(8): e04482. https://doi.org/10.1016/j.heliyon.2020.e04482.
Verma R, Kaushik D. Design and optimization of candesartan loaded self-nanoemulsifying drug delivery system for improving its dissolution rate and pharmacodynamic potential. Drug Deliv. 2020;27(1):756–71. https://doi.org/10.1080/10717544.2020.1760961.
Li S, Madan P, Lin S. Effect of ionization of drug on drug solubilization in SMEDDS prepared using Capmul MCM and caprylic acid. Asian J Pharm Sci. 2017;12(1):73–82. https://doi.org/10.1016/j.ajps.2016.10.001.
Parikh KJ, Sawant KK. Solubilization of vardenafil HCl in lipid-based formulations enhances its oral bioavailability in vivo: a comparative study using Tween - 20 and Cremophor - EL. J Mol Liq. 2019;277:189–99. https://doi.org/10.1016/j.molliq.2018.12.079.
Visetvichaporn V, Kim KH, Jung K, Cho YS, Kim DD. Formulation of self-microemulsifying drug delivery system (SMEDDS) by D-optimal mixture design to enhance the oral bioavailability of a new cathepsin K inhibitor (HL235). Int J Pharm. 2020;573: 118772. https://doi.org/10.1016/j.ijpharm.2019.118772.
Kim YH, Kim YC, Jang D-J, Min KA, Karmacharya J, Nguyen T-T-L, et al. Development of 20(S)-protopanaxadiol-loaded SNEDDS preconcentrate using comprehensive phase diagram for the enhanced dissolution and oral bioavailability. Pharmaceutics. 2020;12(4):362. https://doi.org/10.3390/pharmaceutics12040362.
Cao M, Zhan M, Wang Z, Wang Z, Li XM, Miao M. Development of an orally bioavailable isoliquiritigenin self-nanoemulsifying drug delivery system to effectively treat ovalbumin-induced asthma. Int J Nanomedicine. 2020;15:8945–61. https://doi.org/10.2147/IJN.S269982.
Golwala P, Rathod S, Patil R, Joshi A, Ray D, Aswal VK, et al. Effect of cosurfactant addition on phase behavior and microstructure of a water dilutable microemulsion. Colloids Surf B Biointerfaces. 2020;186: 110736. https://doi.org/10.1016/j.colsurfb.2019.110736.
Qureshi MJ, Mallikarjun C, Kian WG. Enhancement of solubility and therapeutic potential of poorly soluble lovastatin by SMEDDS formulation adsorbed on directly compressed spray dried magnesium aluminometasilicate liquid loadable tablets: a study in diet induced hyperlipidemic rabbits. Asian J Pharm Sci. 2015;10(1):40–56. https://doi.org/10.1016/j.ajps.2014.08.003.
Elkordy AA, Bhangale U, Murle N, Zarara MF. Combination of lactose (as a carrier) with Cremophor® EL (as a liquid vehicle) to enhance dissolution of griseofulvin. Powder Technol. 2013;246:182–6. https://doi.org/10.1016/j.powtec.2013.05.024.
Dholakiya A, Dudhat K, Patel J, Mori D. An integrated QbD based approach of SMEDDS and liquisolid compacts to simultaneously improve the solubility and processability of hydrochlorothiazide. J Drug Deliv Sci Technol. 2021;61:102162. https://doi.org/10.1016/j.jddst.2020.102162.
Wang B, Wang D, Zhao S, Huang X, Zhang J, Lv Y, et al. Evaluate the ability of PVP to inhibit crystallization of amorphous solid dispersions by density functional theory and experimental verify. Eur J Pharm Sci. 2017;96:45–52. https://doi.org/10.1016/j.ejps.2016.08.046.
Ayenew Z, Paudel A, Van den Mooter G. Can compression induce demixing in amorphous solid dispersions? A case study of naproxen–PVP K25. Eur J Pharm Biopharm. 2012;81(1):207–13. https://doi.org/10.1016/j.ejpb.2012.01.007.
Lakshminarayana B, Ashok Kumar KV, Selvaraj M, Satyanarayana G, Ch S. PVP-PS supported ultra-small Pd nanoparticles for the room temperature reduction of 4-nitrophenol. J Environ Chem Eng. 2020;8(4):103899. https://doi.org/10.1016/j.jece.2020.103899.
Vithani K, Jannin V, Pouton CW, Boyd BJ. Colloidal aspects of dispersion and digestion of self-dispersing lipid-based formulations for poorly water-soluble drugs. Adv Drug Deliv Rev. 2019;142:16–34. https://doi.org/10.1016/j.addr.2019.01.008.
Matos RL, Lu T, Prosapio V, McConville C, Leeke G, Ingram A. Coprecipitation of curcumin/PVP with enhanced dissolution properties by the supercritical antisolvent process. J CO2 Util. 2019;30:48–62. https://doi.org/10.1016/j.jcou.2019.01.005.
Wang X, Li Y, Yang X, Yao J. Astragalus polysaccharide reduces inflammatory response by decreasing permeability of LPS-infected Caco2 cells. Int J Biol Macromol. 2013;61:347–52. https://doi.org/10.1016/j.ijbiomac.2013.07.013.
Chen X, Xu L, Guo S, Wang Z, Jiang L, Wang F, et al. Profiling and comparison of the metabolites of diosmetin and diosmin in rat urine, plasma and feces using UHPLC-LTQ-Orbitrap MSn. J Chromatogr B. 2019;1124:58–71. https://doi.org/10.1016/j.jchromb.2019.05.030.
Acknowledgements
We are appreciative to School of Chemistry & Chemical Engineering, Jiangsu University, and School of Materials Science & Engineering, Jiangsu University, for DSC and ATR-FTIR analysis.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 31871810); National Key R&D Program of China (grant number 2018YFE0208600); Six Talent Peaks Scientific Project in Jiangsu Province (grant number SWYY-163); Research Foundation for Distinguished Scholars of Jiangsu University (grant number 15JDG074); and Key Laboratory financial support of Zhenjiang (grant number SS2018004).
Author information
Authors and Affiliations
Contributions
Zhengqing Gu conducted the experiment, analyzed the data, and wrote the manuscript. Yuanyuan Xue carried out the experiment and interpreted the data. Shuang Li carried out the experiment and interpreted the data. Michael Adu-Frimpong reviewed and revised the draft. Ying Xu investigated the project and reviewed the manuscript. Jiangnan Yu and Ximing Xu provided the funding and facilities for study. Yuan Zhu provided the funding, designed the project, supervised the draft, and approved this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gu, Z., Xue, Y., Li, S. et al. Design, Characterization, and Evaluation of Diosmetin-Loaded Solid Self-microemulsifying Drug Delivery System Prepared by Electrospray for Improved Bioavailability. AAPS PharmSciTech 23, 106 (2022). https://doi.org/10.1208/s12249-022-02263-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02263-3