Abstract
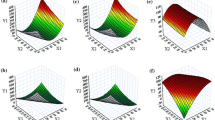
This article aimed to design a new type of supersaturated solid dispersion (NS-SD) loaded with Magnolol (Mag) to raise the oral bioavailability in rats. In the light of the solubility parameters, phase solubility experiments, inhibition precipitation experiment, and in vitro release experiment, Plasdone-630 (PS-630) was selected as the optimum carrier. In addition, Mag-NS-SD was prepared by adding Monoglyceride (MG) and Lecithin High Potency (LHP) into the Mag-S-SD (Mag:PS-630 = 1:3), so as to reduce the dosage of carrier and improve the release rate. Using central composite design of response surface method, the prescription was further optimized. As the optimized condition was Mag:PS-630: MG: LHP = 1:3:0.8:0.266, the drug release rate was the fastest. Besides, after 45 min, the release rate was nearly 100%. The constructed Mag-S-SD and Mag-NS-SD were characterized by powder X-ray diffraction and infrared absorption spectrum. The XRD patterns of Mag-S-SD and Mag-NS-SD indicated that all APIs were amorphous. The IR spectra of Mag-S-SD and Mag-NS-SD demonstrated the existence of hydrogen bonding in the systems. Furthermore, in vivo pharmacokinetic study in rats revealed that compared with Mag and Mag-S-SD, Mag-NS-SD significantly increased the bioavailability (the relative bioavailability was 213.69% and 142.37%, separately). In this study, Mag-NS-SD was successfully prepared, which could improve the oral bioavailability and may increase the clinical application.













Similar content being viewed by others
References
Lee BH, Choi SH, Kim HJ, Park SD, Rhim H, Kim HC, et al. Gintonin absorption in intestinal model systems. J Ginseng Res. 2018;42(1):35–41.
Wenlock MC, Austin RP, Barton P, Davis AM, Leeson PD. A comparison of physiochemical property profiles of development and marketed oral drugs. J Med Chem. 2003;46(7):1250–6.
Mu H, Holm R, Mullertz A. Lipid-based formulations for oral administration of poorly water-soluble drugs. Int J Pharm. 2013;453(1):215–24.
Dhillon B, Goyal NK, Malviya R, Sharma PK. Poorly water soluble drugs: change in solubility for improved dissolution characteristics a review. Glob J Pharmacol. 2014;8(1):26–35.
Park H, Ha ES, Kim MS. Current status of supersaturable self-emulsifying drug delivery systems. Pharmaceutics. 2020;12(4):365.
Higuchi T. Physical chemical analysis of percutaneous absorption process from creams and ointments. Jsoccosmetchem. 1960;11:85–97.
Brouwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J Pharm Sci. 2009;98(8):2549–72.
Guzma H, Tawa M, Zhang Z, Ratanabanangkoon P, Remenar J. Spring and parachute approach to designing solid celecoxib formulations having enhanced oral absorption. AAPS J. 2004;6:21–9.
Xu S, Dai WG. Drug precipitation inhibitors in supersaturable formulations. Int J Pharm. 2013;453(1):36–43.
Gao P, Rush BD, Pfund WP. Development of a supersaturable SEDDS (S-SEDDS) formulation of paclitaxel with improved oral bioavailability. J Pharm Sci. 2003;92(12):2386–98.
Huang Y, Dai WG. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm Sin B. 2013;4(1):18–25.
Urbanetz NA, Lippold BC. Solid dispersions of nimodipine and polyethylene glycol 2000: dissolution properties and physico-chemical characterisation. Eur J Pharm Biopharm. 2005;59(1):107–18.
Tsunashima D, Yamashita K, Ogawara KI, Sako K, Higaki K. Preparation of extended release solid dispersion formulations of tacrolimus using ethylcellulose and hydroxypropylmethylcellulose by solvent evaporation method. J Pharm Pharmacol. 2016;68(3):316–23.
Pinto JMO, Leão AF, Riekes MK, França MT, Stulzer HK. HPMCAS as an effective precipitation inhibitor in amorphous solid dispersions of the poorly soluble drug candesartan cilexetil. Carbohydr Polym. 2017;2017:199–206.
Zhu C, Gong S, Ding J, Yu M, Ahmad E, Feng Y, et al. Supersaturated polymeric micelles for oral silybin delivery: the role of the Soluplus–PVPVA complex. Acta Pharm Sin B. 2019;9(01):107–17.
Xia D, Yu H, Tao J, Zeng J, Zhu Q, Zhu C, et al. Supersaturated polymeric micelles for oral cyclosporine A delivery: the role of Soluplus–sodium dodecyl sulfate complex. Colloids Surf B Biointerfaces. 2016;141:301–10.
Suzuki H, Sunada H. Some factors influencing the dissolution of solid dispersions with nicotinamide and hydroxypropylmethylcellulose as combined carriers. Chem Pharm Bull. 1998;46(6):1015–20.
Yongfei L. Preparation and dissolution of resveratrol S-SMEDDS. Technol Wind. 2019;36(36):135–7.
Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Euro J Pharm Sci. 2000;11:93–8.
Guilan Q, Boyi N, Vikramjeet S, Yixian Z, Chuan-Yu W, Xin P, et al. Supersaturable solid self-microemulsifying drug delivery system: precipitation inhibition and bioavailability enhancement. Int J Nanomed. 2017;12:8801–11.
Li P, Hynes SR, Haefele TF, Pudipeddi M, Royce AE, Serajuddin ATM. Development of clinical dosage forms for a poorly water-soluble drug II: formulation and characterization of a novel solid microemulsion preconcentrate system for oral delivery of a poorly water-soluble drug. J Pharm Sci. 2010;98(5):1750–64.
Chavan RB, Modi SR, Bansal AK. Role of solid carriers in pharmaceutical performance of solid supersaturable SEDDS of celecoxib. Int J Pharm. 2015;495(1):374–84.
Agarwal V, Siddiqui A, Ali H, Nazzal S. Dissolution and powder flow characterization of solid self-emulsified drug delivery system (SEDDS). Int J Pharm. 2008;366(1–2):44–52.
Lin MH, Chen MC, Chen TH, Chang HY, Chou TC. Magnolol ameliorates lipopolysaccharide-induced acute lung injury in rats through PPAR-γ-dependent inhibition of NF-kB activation. Int Immunopharmacol. 2015;28(1):270–8.
Lin CF, Hwang TL, Al-Suwayeh SA, Huang YL, Fang JY. Maximizing dermal targeting and minimizing transdermal penetration by magnolol/honokiol methoxylation. Int J Pharmaceut. 2013;445(1):153–62.
Pacult J, Rams-Baron M, Chrzaszcz B, et al. Effect of polymer chain length on the physical stability of amorphous drug-polymer blends at ambient pressure. Mol Pharm. 2018;15(7):2807–15.
Li Y, Lu M, Wu C. PVP VA64 as a novel release-modifier for sustained-release mini-matrices prepared via hot melt extrusion. Drug Deliv Transl Res. 2018;8(6):1670–8.
Xia D, Yu H, Tao J, Zeng J, Zhu Q, Zhu C, et al. Supersaturated polymeric micelles for oral cyclosporine A delivery: the role of Soluplus-sodium dodecyl sulfate complex. Colloids Surf B. 2016;141:301–10.
Yu H, Xia D, Zhu Q, Zhu C, Chen D, Gan Y. Supersaturated polymeric micelles for oral cyclosporine A delivery. Eur J Pharm Biopharm. 2013;85(3):1325–36.
Hancock BC, York P, Rowe RC. The use of solubility parameters in pharmaceutical dosage form design. Int J Pharm. 1997;148(1):1–21.
Kuentz M, Holm R, Elder DP. Methodology of oral formulation selection in the pharmaceutical industry. Eur J Pharm Sci. 2016;87:136–63.
Forster A, Hempenstall J, Tucker I, Rades T. Selection of excipients for melt extrusion with two poorly water-soluble drugs by solubility parameter calculation and thermal analysis. Int J Pharm. 2001;226(1–2):147–61.
Li PLX, Zhou H. Prediction method for the compatibility between drug and carrier. Chin J New Drugs. 2009;18(3):262–71.
Zhang HSL, Wang Y. Research progress on excipients of solid dispersion prepared by hot-melt extrusion technique. Drug Clinic. 2014;29(5):557–63.
Lu Y, Xu Z. Optimization of curcumin solid dispersion formulation and in vitro dissolution evaluation. Herb Depot. 2021;30(2):31–7.
Fedors RF. A method for estimating both the solubility parameters and molar volumes of liquids. Polym Eng Sci. 1974;14(2):147–54.
El-Banna HM, Daabis NA, Mortada LM, Abd-Elfattah S. Physicochemical study of drug binary systems. Part 3: tolbutamide-urea and tolbutamide-mannitol systems. Pharmazie. 1976;30(12):788–92.
Nakai Y, Yamamoto K, Oguchi T, et al. Determination of solubility parameters for solid medicinals and excipients. J Pharmacobio-dyn. 1989;12(2):20–3.
Rhodes CT. Drug development and industrial pharmacy. Drug Dev Commun. 1995;21(20):2263–85.
Bloch DW, Elegakey MA, Speiser PP. Solid dispersion of chlorthalidone in urea phase diagram and dissolution characteristics. Pharm Acta Helv. 1982;57(8):231–5.
Li J, Jiang ZT. Preparation and bioavailability of magnolol solid dispersions. Chin Trad Herb Drugs. 2019;14(50):3337–44.
Bavishi DD, Borkhataria CH. Spring and parachute: how cocrystals enhance solubility. Prog Cryst Growth Charact Mater. 2016;62(3):1–8.
Zhu C, Gong S, Ding J, Yu M, Ahmad E, Feng Y, et al. Supersaturated polymeric micelles for oral silybin delivery: the role of the Soluplus-PVPVA complex. Acta Pharm Sin B. 2019;9(1):107–17.
Yamashita T, Ozaki S, Kushida I. Solvent shift method for anti-precipitant screening of poorly soluble drugs using biorelevant medium and dimethyl sulfoxide. Int J Pharm. 2011;419(1):170–4.
Ahn J-H, Kim Y-P, Lee Y-M, Seo E-M, Lee K-W, Kim H-S. Optimization of microencapsulation of seed oil by response surface methodology. Food Chem. 2008;107(1):98–105.
Das SS, Singh A, Kar S, Ghosh R, Pal M, Fatima M, et al. Application of QbD framework for development of self-emulsifying drug delivery systems. In: Pharmaceutical Quality by Design. 2019. p. 297–350.
Dian L, Yu E, Chen X, Wen X, Zhang Z, Qin L, et al. Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles. Nanoscale Res Lett. 2014;9(1):2406.
Wang L, Huang T, Zeng J. Application progress of copolymer Soluplus® in new pharmaceutical formulations and technologies. Chin Pharm. 2016;27(19):2703–7.
Jiang WLY. Progress of applications of copovidone to pharmaceutical preparations. Chin J Pharm. 2015;46(8):898–903.
Ma F, Li X, Yin J, Ma L, Li D. Optimisation of double-enzymatic extraction of arabinoxylan from fresh corn fibre. J Food Sci Technol. 2020;57:1–11.
Dilpreet S, Manisha S, K TA, Neena B. Evaluation of bio-mechanistic behavior of liquid self-microemulsifying drug delivery system in biorelevant media. Assay Drug Dev Technol. 2020;19:85–96.
Ujhelyi Z, Vecsernyés M, Fehér P, Kósa D, Arany P, Nemes D, et al. Physico-chemical characterization of self-emulsifying drug delivery systems. Drug Discov Today Technol. 2018;27:81–6.
Zhong L, Li X, Liu L, Liao Y, Tang H, Xie L, et al. Research progress on application and curing of self-microemulsion in traditional Chinese medicine preparation. Pharm Clinic Chin Mater Med. 2019;10(2):53-8+64.
Gong L, Liao G, Chen Q, Luan H, Feng Y. Swollen surfactant micelles: properties and applications. Acta Phys Chim Sin. 2019;35(8):816–28.
Chen P. Molecular interfacial phenomena of polymers and biopolymers. Mater Today. 2005;8(11):62–3.
Ding T. Application of EXCEL in statistical test of bioequivalence. Chin Pharm. 2007;2007(01):93–4.
Han W, Jiang J. Overview of statistical evaluation methods for bioequivalence. Chin J Health Stat. 2010;27(04):441–5.
Sun W, Sun R. Two one side t-test for computational simplicity of bioavailability equivalence. Chin J Clin Pharmacol Ther. 1997;04:279–82.
Acknowledgements
We are grateful that this work was supported by grants from 2020 Liaoning Provincial Department of Education Scientific Research Funding Project—Key Research Project (NO. 2020LZD02) and Open Fund of the State Key Laboratory of New Technology of Chinese Medicine Pharmaceutical Process (No. SKL2020Z0206).
Author information
Authors and Affiliations
Contributions
Jing Zhao: conceptualization, methodology, formal analysis, investigation, resources, data curation, writing—review and editing, visualization, supervision, project administration; Pan Gao: conceptualization, methodology, formal analysis, investigation, resources, data curation, writing—original draft, visualization, supervision, project administration; Chengqiao Mu: conceptualization, formal analysis, investigation, data curation; Jingqi Ning: conceptualization, methodology, data acquisition, formal analysis; Wenbin Deng: conceptualization, methodology, formal analysis, data curation; Dongxu Ji: literature review, writing—original draft, data curation; Haowei Sun: writing—original draft, visualization, supervision; Xiangrong Zhang: conceptualization, methodology, formal analysis, writing—review and editing, project administration; Xinggang Yang: conceptualization, methodology, formal analysis, writing—review and editing, project administration, funding acquisition.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Zhao and Pan Gao have contributed equally to this work and share first authorship.
Theme: Advancements in Amorphous Solid Dispersions to Improve Bioavailability
Rights and permissions
About this article
Cite this article
Zhao, J., Gao, P., Mu, C. et al. Preparation and Evaluation of Novel Supersaturated Solid Dispersion of Magnolol. AAPS PharmSciTech 23, 97 (2022). https://doi.org/10.1208/s12249-022-02251-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02251-7