Abstract
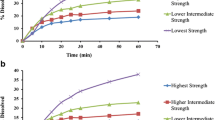
Dissolution profile comparison among different formulations plays a critical role during new drug as well as generic product development. In the generic product development, dissolution profile comparison is a mandate for biowaivers (BCS-based, for lower strengths and IVIVC-based biowaivers) and also from quality control perspective. Even though traditionally similarity factor or f2 is used as a metric for dissolution profile comparison, it comes with multiple limitations and requirements (e.g., number of time points and variability). To overcome this, regulatory agencies suggested model-independent (e.g., MSD) and model-dependent (e.g., zero order, Weibull) dissolution profile comparison methods. Although most of regulatory guidance documents mention about such approaches, their usage in reality is limited probably due to lack of clear, detailed, and step-wise procedure. In this context, the present article describes simplistic yet detailed procedures of dissolution profile comparison with case studies covering generic product development scenario’s from a regulatory perspective. Detailed review of regulatory guidances from various agencies was made along with examples of such approaches in regulatory submissions. Data from three formulations—Formulations A, B, and C—were utilized to perform dissolution profile comparison using MSD, zero-order, and Weibull release profile–based comparisons. Dissolution profile comparisons were made using all of these three approaches complying with regulatory requirements. These examples demonstrated value and utility of these approaches and the simplified and detailed procedure explained in this manuscript can be adapted for generic product applications.





Similar content being viewed by others
Abbreviations
- API:
-
active pharmaceutical ingredient
- BCS:
-
biopharmaceutics classification system
- IVIVC:
-
in vitro and in vivo correlation
- MSD:
-
multivariate statistical distance
- T2EQ:
-
T^2test for equivalence
- USFDA:
-
United States Food and Drug Administration
- EMA:
-
European Medicines Agency
- ANVISA:
-
Agencia Nacional de Vigilancia Sanitaria
- MOH:
-
Ministry of Health
- RSD:
-
relative standard deviation
- RLD:
-
reference listed drug
- QC:
-
quality control
- CR:
-
controlled release
References
USFDA BCS Based biowaiver guidance. 2021. https://www.fda.gov/media/148472/download#:~:text=The%20BCS%2Dbased%20biowaiver%20is,based%20biowaiver%20in%20this%20guidance. Accessed 10 Oct 2021.
USFDA IVIVC guidance. 1997. https://www.fda.gov/media/70939/download. .
USFDA Dissolution guidance. 1997. https://www.fda.gov/media/70936/download. .
USFDA SUPAC MR guidance. 1997. https://www.fda.gov/media/70956/download. Accessed 10 Oct 2021.
EMA Bioequivalence guidance. 2010. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf. Accessed 10 Oct 2021.
Shah VP, Tsong Y, Sathe P, Liu JP. In vitro dissolution profile comparison—statistics and analysis of the similarity factor, f2. Pharm Res. 1998;15:889–96. https://doi.org/10.1023/a:1011976615750.
Hoffelder T. Comparison of dissolution profiles: a statistician’s perspective. Ther Innov Regul Sci. 2018;52(4):423–9. https://doi.org/10.1177/2168479017749230.
TGA Minor variations to prescription medicines, Appendix 1. Variation change types, chemical entities. 2017. https://www.tga.gov.au/sites/default/files/guidance-minor-variations-chemical-entities.pdf. Accessed 10 Oct 2021.
ASEAN guidance for conduct of bioequivalence studies. 2015. https://asean.org/wp-content/uploads/2012/10/BE_Guideline_FinalMarch2015_endorsed_22PPWG.pdf. Accessed 10 Oct 2021.
CDE. Determination and comparison of dissolution curves of ordinary solid preparations guideline. 2016. https://www.nmpa.gov.cn/zhuanti/ypqxgg/ggzhcfg/20160318210001633.html. Accessed 10 Oct 2021.
Health Canada, Post notice of compliance changes in quality document. 2019. https://www.canada.ca/content/dam/hc-sc/documents/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/post-notice-compliance-changes/quality-document/quality-document.pdf. Accessed 10 Oct 2021.
Brazil / ANVISA, Realization of pharmaceutical equivalence studies and comparative dissolution profiles. 2010. RDC 31/2010.
Russia, On approval of Rules for Medicinal Products Bioequivalence Studies within the Eurasian Economic Union. 2016.
Flubiprofen, Public assessment report scientific discussion. 2017. https://cima.aemps.es/cima/pdfs/ipe/81909/IPE_81909.pdf. Accessed 10 Oct 2021.
Dasatinib, Public assessment report scientific discussion. 2018. https://mri.cts-mrp.eu/Human/Downloads/NL_H_4003_004_PAR.pdf. Accessed 10 Oct 2021.
Erlotinib, Public assessment report scientific discussion. 2019. https://mri.cts-mrp.eu/Human/Downloads/NL_H_4092_003_PAR.pdf. Accessed 10 Oct 2021.
Rivaroxaban Assessment report. 2020. https://www.ema.europa.eu/en/documents/assessment-report/rivaroxaban-accord-epar-public-assessment-report_en.pdf. Accessed 10 Oct 2021.
Deferasirox Assessment report. 2019. https://wizmed.com/emar/deferasirox-accord-epar-public-assessment-report_en.pdf. Accessed 10 Oct 2021.
EMA questions and answers on the adequacy of Mahalanobis distance to assess the comparability of drug dissolution profiles. 2018. https://www.ema.europa.eu/en/documents/scientific-guideline/question-answer-adequacy-mahalanobis-distance-assess-comparability-drug-dissolution-profiles_en.pdf. Accessed 10 Oct 2021.
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, Xie S. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12(3):263–71. https://doi.org/10.1208/s12248-010-9185-1.
Tsong Y, Hammerstrom T, Sathe P, Shah VP. Statistical assessment of mean differences between two dissolution data sets. Ther Innov Regul Sci. 1996;30:1105–12. https://doi.org/10.1177/009286159603000427.
Sathe PM, Tsong Y, Shah VP. In vitro dissolution profile comparison. Statistics and analysis model dependent approach. Pharm Res. 1996;13(12):1799–803. https://doi.org/10.1023/a:1016020822093.
Papadopoulou V, Kosmidis K, Vlachou M, Macheras P. On the use of the Weibull function for the discernment of drug release mechanisms. Int J Pharm. 2006;309(1-2):44–50. https://doi.org/10.1016/j.ijpharm.2005.10.044.
Acknowledgements
Authors would like to thank Dr. Reddy’s Laboratory for providing an opportunity to publish this work. We would like to thank statistician Daka Krishna Reddy for inputs in the manuscript.
Author information
Authors and Affiliations
Contributions
Sivacharan Kollipara—concept and design, writing manuscript; Rajkumar Boddu—concept and design, writing manuscript; Tausif Ahmed—concept and design, manuscript review, approval for version to be published; Siddharth Chachad—manuscript review, approval for version to be published
Corresponding author
Ethics declarations
Conflict of Interest
All the authors are employees of Dr. Reddy’s laboratory and report no conflicts interest. The authors alone are responsible for the content and writing of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Kollipara, S., Boddu, R., Ahmed, T. et al. Simplified Model-Dependent and Model-Independent Approaches for Dissolution Profile Comparison for Oral Products: Regulatory Perspective for Generic Product Development. AAPS PharmSciTech 23, 53 (2022). https://doi.org/10.1208/s12249-021-02203-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02203-7