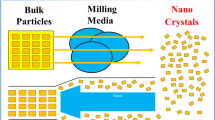
Abstract
Drugs are referred to as drug nanocrystals when they exist as nanoscale crystal structures. This kind of nanocarrier has been widely utilized to increase the solubility and absorption for poorly aqueous soluble drugs after oral administration, or prolong the drug circulation when intravenous administration. The systemic cytotoxicity caused by antitumor drugs usually come from the nonspecific drug distribution. To solve the disadvantage of poor targetability, drug nanocrystals for tumor targeted delivery have been developed in recent years. In this review, the targeting mechanisms of various surface modified drug nanocrystals are introduced with the focus on passive targeting, active targeting and stimuli-responsive targeting in details. Function and application of common surface modified materials are also discussed.
Graphical abstract






Similar content being viewed by others
Abbreviations
- Topo I:
-
topoisomerase I
- PEG:
-
polyethylene glycol
- PCL:
-
polycaprolactone
- mPEG:
-
Polyethylene glycol monomethyl ether
- HA:
-
hyaluronic acid
- CLT-NCs:
-
cilostazol nanocrystals
- DW:
-
distilled water
- CSOA-DOX NCs:
-
doxorubicin nanocrystals modified with chondroitin sulfate
- DOX/PEG-PCL:
-
doxorubicin loaded micelles modified with polycaprolactone and polyethylene glycol
- PTX-NCs-Gel:
-
paclitaxel nanocrystals-hydrogel system
- EPR:
-
high permeability and retention
- IDM/PTX NCs:
-
indomethacin modified paclitaxel nanocrystals
- MTO NCs:
-
mitoxantrone nanocrystals
- RGD:
-
Arginine-Glycine-Aspartic acid
- CS:
-
chondroitin sulfate
- HAase:
-
hyaluronidase
- FA:
-
Folic acid
- HCPT:
-
hydroxycamptothecin
- HCPT NCs@AuNPs-Zein-PFA:
-
Au nanoparticles as the core and FA-conjugated amphiphilic Zein-PFA as the shell with encapsulation of HCPT nanocrystals
- PTX NCs@PDA-PEG-RGD:
-
paclitaxel nanocrystals with PEG and RGD peptide
- Tf R:
-
transferrin receptors
- DTX:
-
docetaxel
- Tf-PTX-NCs:
-
transferrin-modified paclitaxel nanocrystals
- CNS:
-
central nervous system
- EGFR:
-
epidermal growth factor receptor
- PTX@PCL-PEG-Herceptin:
-
Herceptin-conjugated, paclitaxel loaded PCL-PEG worm-like nanocrystals
- ZnS:Mn NCs:
-
Mn-doped zinc sulphide nanocrystals
- DAI:
-
the disease activity index
- MMPs:
-
matrix metalloproteinases
- COX-2:
-
cyclooxygenase-2
- PKD1:
-
protein kinase D1
- MNCs:
-
magnetic nanocrystals
- ACMF:
-
alternating current magnetic field
- ROS:
-
reactive oxygen species
- GSH:
-
glutathione
- OA:
-
oleic acid
- NIR:
-
near-infrared
- PTT:
-
photothermal therapy
- PDT:
-
photodynamic therapy
- LCST:
-
low critical solution temperature
- PVA:
-
polyvinyl alcohol
- CAP1AG4CH5@CUNCs:
-
chitosan (CH)/alginate (AG)/cellulose acetate phthalate (CAP)multi-layer curcumin nanocrystals
- CNC-SS-PD3/pCMV-CD/5-FC:
-
cytosine deaminase/5-fluorocytosine loaded on cellulose nanocrystals (CNCs) made from natural cotton with poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) linked by disulfide bonds
- CeVO4/Au NCs:
-
semiconductor nanomaterials through loading plasmonic metal nanoparticle
- TMZ-Bi2O3@PVA:
-
Bi2O3 nanocrystals fixed in polyvinyl alcohol (PVA) nanogels with temozolomide
- RGD-RBC-DTX NCs:
-
c(RGDyK) peptide modified and red blood cell membrane encapsulated docetaxel nanocrystals.
References
Malamatari M, Taylor KMG, Malamataris S, Douroumis D, Kachrimanis K. Pharmaceutical nanocrystals: production by wet milling and applications. Drug Discov Today. 2018;23(3):534–47. https://doi.org/10.1016/j.drudis.2018.01.016.
Liang Y, Fu X, Du C, Xia H, Lai Y, Sun Y. Enzyme/pH-triggered anticancer drug delivery of chondroitin sulfate modified doxorubicin nanocrystal. Artif Cell Nanomed Biotechnol. 2020;48(1):1114–24. https://doi.org/10.1080/21691401.2020.1813741.
Mao Y, Liu J, Shi T, Chen G, Wang S. A Novel Self-Assembly Nanocrystal as Lymph Node-Targeting Delivery System: Higher Activity of Lymph Node Targeting and Longer Efficacy Against Lymphatic Metastasis. AAPS PharmSciTech. 2019;20(7):292. https://doi.org/10.1208/s12249-019-1447-3.
Zhang HW, Dang Q, Zhang ZW, Wu FS. Development, characterization and evaluation of doxorubicin nanostructured lipid carriers for prostate cancer. J BUON. 2017;22(1):102–11.
Kim HM, Lee GH, Kuh HJ, Kwak BK, Lee J. Liposomal doxorubicin-loaded chitosan microspheres capable of controlling release of doxorubicin for anti-cancer chemoembolization: in vitro characteristics. J Drug Deliv Sci Technol. 2013;23(3):283–6.
Wang J, Muhammad N, Li T, Wang H, Liu Y, Liu B, Zhan H. Hyaluronic Acid-Coated Camptothecin Nanocrystals for Targeted Drug Delivery to Enhance Anticancer Efficacy. Mol Pharm. 2020;17(7):2411–25. https://doi.org/10.1021/acs.molpharmaceut.0c00161.
Gan MY, Zhang WP, Wei SJ, Dang HW. The influence of mPEG-PCL and mPEG-PLGA on encapsulation efficiency and drug-loading of SN-38 NPs. Artif Cell Nanomed Biotechnol. 2017;45(2):389–97. https://doi.org/10.3109/21691401.2016.1167700.
Hou Z, Zhou S, Cui F, Hang Y, Yi Y. Preparation and characterization of hydroxycamptothecin-loaded Poly(D,L-lactic acid) nanoparticles with high drug loading capacity. In: Zhang H, Jin D, editors. Advanced Research on Material Engineering, Chemistry, Bioinformatics Ii2012. p. 503.
Lin YA, Cheetham AG, Zhang PC, Ou YC, Li YG, Liu GS, Hermida-Merino D, Hamley IW, Cui H. Multiwalled Nanotubes Formed by Catanionic Mixtures of Drug Amphiphiles. Acs Nano. 2014;8(12):12690–700. https://doi.org/10.1021/nn505688b.
Fang S, Hou YP, Ling LB, Wang DQ, Ismail M, Du YW, et al. Dimeric camptothecin derived phospholipid assembled liposomes with high drug loading for cancer therapy. Colloids Surf B. 2018;166:235–44. https://doi.org/10.1016/j.colsurfb.2018.02.046.
Sharma S, Verma A, Pandey G, Mittapelly N, Mishra PR. Investigating the role of Pluronic-g-Cationic polyelectrolyte as functional stabilizer for nanocrystals: Impact on Paclitaxel oral bioavailability and tumor growth. Acta Biomater. 2015;26:169–83. https://doi.org/10.1016/j.actbio.2015.08.005.
Liu Y, Huang L, Liu F. Paclitaxel Nanocrystals for Overcoming Multidrug Resistance in Cancer. Mol Pharm. 2010;7(3):863–9. https://doi.org/10.1021/mp100012s.
Lv PP, Wei W, Yue H, Yang TY, Wang LY, Ma GH. Porous Quaternized Chitosan Nanoparticles Containing Paclitaxel Nanocrystals Improved Therapeutic Efficacy in Non-Small-Cell Lung Cancer after Oral Administration. Biomacromolecules. 2011;12(12):4230–9. https://doi.org/10.1021/bm2010774.
Filippousi M, Papadimitriou SA, Bikiaris DN, Pavlidou E, Angelakeris M, Zamboulis D, Tian H, van Tendeloo G. Novel core-shell magnetic nanoparticles for Taxol encapsulation in biodegradable and biocompatible block copolymers: Preparation, characterization and release properties. Int J Pharm. 2013;448(1):221–30. https://doi.org/10.1016/j.ijpharm.2013.03.025.
Liang N, Sun SP, Hong J, Tian JZ, Fang L, Cui FD. In vivo pharmacokinetics, biodistribution and antitumor effect of paclitaxel-loaded micelles based on alpha-tocopherol succinate-modified chitosan. Drug Deliv. 2016;23(8):2651–60. https://doi.org/10.3109/10717544.2015.1045103.
Choi JS. Design of Cilostazol Nanocrystals for Improved Solubility. J Pharm Innov. 2019;15(3):416–23. https://doi.org/10.1007/s12247-019-09391-7.
Zhang X, Li L, Gao X, Zhang H, Gao J, Du YM, et al. In Vitro Evaluation of Quercetin Nanocrystals with Different Particle Sizes. J Nanosci Nanotechnol. 2020;20(10):6469–74. https://doi.org/10.1166/jnn.2020.18580.
Kakran M, Shegokar R, Sahoo NG, Al Shaal L, Li L, Mueller RH. Fabrication of quercetin nanocrystals: Comparison of different methods. Eur J Pharm Biopharm. 2012;80(1):113–21. https://doi.org/10.1016/j.ejpb.2011.08.006.
He X, Song H. Study on preparation and characteristics of naringenin nanocrystals in vitro. Chin J Hosp Pharm. 2016;36(4):289–93.
Ige PP, Baria RK, Gattani SG. Fabrication of fenofibrate nanocrystals by probe sonication method for enhancement of dissolution rate and oral bioavailability. Colloids Surf B. 2013;108:366–73. https://doi.org/10.1016/j.colsurfb.2013.02.043.
Desai SK, Mondal D, Bera S. First-line anti-tubercutilosis drugs-loaded starch nanocrystals for combating the threat of M. tuberculosis H37Rv strain. Carbohydr Res. 2020;495:108070. https://doi.org/10.1016/j.carres.2020.108070.
George M, Ghosh I. Identifying the correlation between drug/stabilizer properties and critical quality attributes (CQAs) of nanosuspension formulation prepared by wet media milling technology. Eur J Pharm Sci. 2013;48(1-2):142–52. https://doi.org/10.1016/j.ejps.2012.10.004.
Yue P, Li Y, Wan J, Yang M, Zhu W, Wang C. Study on formability of solid nanosuspensions during nanodispersion and solidification: I. Novel role of stabilizer/drug property. Int J Pharm. 2013;454(1):269–77. https://doi.org/10.1016/j.ijpharm.2013.06.050.
Singhal D, Curatolo W. Drug polymorphism and dosage form design: a practical perspective. Adv Drug Del Rev. 2004;56(3):335–47. https://doi.org/10.1016/j.addr.2003.10.008.
Lai F, Sinico C, Ennas G, Marongiu F, Marongiu G, Fadda AM. Diclofenac nanosuspensions: Influence of preparation procedure and crystal form on drug dissolution behaviour. Int J Pharm. 2009;373(1-2):124–32. https://doi.org/10.1016/j.ijpharm.2009.01.024.
Lestari MLAD, Mueller RH, Moeschwitzer JP. Systematic Screening of Different Surface Modifiers for the Production of Physically Stable Nanosuspensions. J Pharm Sci. 2015;104(3):1128–40. https://doi.org/10.1002/jps.24266.
Van Eerdenbrugh B, Vermant J, Martens JA, Froyen L, Van Humbeeck J, Augustijns P, et al. A Screening Study of Surface Stabilization during the Production of Drug Nanocrystals. J Pharm Sci. 2009;98(6):2091–103. https://doi.org/10.1002/jps.21563.
Lee J, Choi JY, Park CH. Characteristics of polymers enabling nano-comminution of water-insoluble drugs. Int J Pharm. 2008;355(1-2):328–36. https://doi.org/10.1016/j.ijpharm.2007.12.032.
Zhang W, Yu L, Ji T, Wang C. Tumor Microenvironment–responsivepeptide-based supramolecular drug delivery system. Front Chem. 2020;8. https://doi.org/10.3389/fchem.2020.00549.
Duan X, Li Y. Physicochemical Characteristics of Nanoparticles Affect Circulation, Biodistribution, Cellular Internalization, and Trafficking. Small. 2013;9(9-10):1521–32. https://doi.org/10.1002/smll.201201390.
Hollis CP, Weiss HL, Leggas M, Evers BM, Gemeinhart RA, Li T. Biodistribution and bioimaging studies of hybrid paclitaxel nanocrystals: lessons learned of the EPR effect and image-guided drug delivery. J Control Release. 2013;172(1):12–21. https://doi.org/10.1016/j.jconrel.2013.06.039.
Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55(17):3752–6.
Zhang C, Long L, Xiong Y, Wang C, Peng C, Yuan Y, Liu Z, Lin Y, Jia Y, Zhou X, Li X. Facile Engineering of Indomethacin-Induced Paclitaxel Nanocrystal Aggregates as Carrier-Free Nanomedicine with Improved Synergetic Antitumor Activity. ACS Appl Mater Interfaces. 2019;11(10):9872–83. https://doi.org/10.1021/acsami.8b22336.
Mei X, Li M, Gao G, Ma S, Liu C, Hu X. Initial analysis of nano carrier targeted drug delivery system: misunderstanding, barriers and strategy. Scientia Sinica Vitae. 2016;46(11):1249–58.
Angelopoulou A, Kolokithas Ntoukas A, Fytas C, Avgoustakis K. Folic acid-functionalized, condensed magnetic nanoparticles for targeted delivery of doxorubicin to tumor cancer cells overexpressing the folate receptor. ACS Omega. 2019;4(26):22214–27. https://doi.org/10.1021/acsomega.9b03594.
Wang H, Zhu W, Huang Y, Li Z, Jiang Y, Xie Q. Facile encapsulation of hydroxycamptothecin nanocrystals into zein-based nanocomplexes for active targeting in drug delivery and cell imaging. Acta Biomater. 2017;61:88–100. https://doi.org/10.1016/j.actbio.2017.04.017.
Huang ZG, Lv FM, Wang J, Cao SJ, Liu ZP, Liu Y, Lu WY. RGD-modified PEGylated paclitaxel nanocrystals with enhanced stability and tumor-targeting capability. Int J Pharm. 2019;556:217–25. https://doi.org/10.1016/j.ijpharm.2018.12.023.
Xie J, Yan C, Yan Y, Chen L, Song L, Zang F, An Y, Teng G, Gu N, Zhang Y. Multi-modal Mn-Zn ferrite nanocrystals for magnetically-induced cancer targeted hyperthermia: a comparison of passive and active targeting effects. Nanoscale. 2016;8(38):16902–15. https://doi.org/10.1039/c6nr03916b.
Khoo TC, Tubbesing K, Rudkouskaya A, Rajoria S, Sharikova A, Barroso M, et al. Quantitative label-free imaging of iron-bound transferrin in breast cancer cells and tumors. Redox Biol. 2020;36:101617. https://doi.org/10.1016/j.redox.2020.101617.
Lu Y, Wang Z, Li T, McNally H, Park K, Sturek M. Development and evaluation of transferrin-stabilized paclitaxel nanocrystal formulation. J Control Release. 2014;176:76–85. https://doi.org/10.1016/j.jconrel.2013.12.018.
Choi JS, Park JS. Development of docetaxel nanocrystals surface modified with transferrin for tumor targeting. Drug Des Devel Ther. 2016;11:17–26. https://doi.org/10.2147/dddt.s122984.
Qi Y, Zhang T, Jing C, Liu S, Zhang C, Alvarez PJJ, Chen W. Nanocrystal facet modulation to enhance transferrin binding and cellular delivery. Nat Commun. 2020;11(1):1262. https://doi.org/10.1038/s41467-020-14972-z.
Sun W, Du Y, Liang X, Yu C, Fang J, Lu W, et al. Synergistic triple-combination therapy with hyaluronic acid-shelledPPy/CPT nanoparticles results in tumor regression and prevents tumor recurrence and metastasis in 4T1 breast cancer. Biomater. 2019;217:119264. https://doi.org/10.1016/j.biomaterials.2019.119264.
Chen ZJ, He N, Chen MH, Zhao L, Li XH. Tunable conjugation densities of camptothecin on hyaluronic acid for tumor targeting and reduction-triggered release. Acta Biomate. 2016;43:195–207. https://doi.org/10.1016/j.actbio.2016.07.020.
Liu P, Chen N, Yan L, Gao F, Ji D, Zhang S, Zhang L, Li Y, Xiao Y. Preparation, characterisation and in vitro and in vivo evaluation of CD44-targeted chondroitin sulphate-conjugated doxorubicin PLGA nanoparticles. Carbohydr Polym. 2019;213:17–26. https://doi.org/10.1016/j.carbpol.2019.02.084.
Zhang BZ, Cheng GG, Zheng MB, Han JY, Wang BB, Li MX, Chen J, Xiao T, Zhang J, Cai L, Li S, Fan X. Targeted delivery of doxorubicin by CSA-binding nanoparticles for choriocarcinoma treatment. Drug Deliv. 2018;25(1):461–71. https://doi.org/10.1080/10717544.2018.1435750.
Ma MF, Shang WQ, Jia RX, Chen RJ, Zhao M, Wang CQ, Tian M, Yang S, Hao A. A novel folic acid hydrogel loading beta-cyclodextrin/camptothecin inclusion complex with effective antitumor activity. J Incl Phenom Macrocycl Chem. 2020;96(1-2):169–79. https://doi.org/10.1007/s10847-019-00962-2.
Mansur AAP, Amaral-Junior JC, Carvalho SM, Carvalho IC, Mansur HS. Cu-In-S/ZnS@carboxymethylcellulose supramolecular structures: Fluorescent nanoarchitectures for targeted-theranostics of cancer cells. Carbohydr Polym. 2020;247:116703. https://doi.org/10.1016/j.carbpol.2020.116703.
Hao HQ, Ma QM, He F, Yao P. Doxorubicin and Fe3O4 loaded albumin nanoparticles with folic acid modified dextran surface for tumor diagnosis and therapy. J Mater Chem B. 2014;2(45):7978–87. https://doi.org/10.1039/c4tb01359j.
Yan Y, Wang RZ, Hu Y, Sun RY, Song T, Shi XY, Yin S. Stacking of doxorubicin on folic acid-targeted multiwalled carbon nanotubes for in vivo chemotherapy of tumors. Drug Deliv. 2018;25(1):1607–16. https://doi.org/10.1080/10717544.2018.1501120.
Liang XH, Fan J, Zhao YY, Cheng M, Wang XJ, Jin RY, Sun T. A targeted drug delivery system based on folic acid-functionalized upconversion luminescent nanoparticles. J Biomater Appl. 2017;31(9):1247–56. https://doi.org/10.1177/0885328217701289.
Peng J, Chen J, Xie F, Bao W, Xu H, Wang H, Xu Y, du Z. Herceptin-conjugated paclitaxel loaded PCL-PEG worm-like nanocrystal micelles for the combinatorial treatment of HER2-positive breast cancer. Biomater. 2019;222:119420. https://doi.org/10.1016/j.biomaterials.2019.119420.
Büyükköroğlu G, Şenel B, Gezgin S, Dinh T. The simultaneous delivery of paclitaxel and Herceptin® using solid lipid nanoparticles: In vitro evaluation. J Drug Deliv Sci Technol. 2016;35:98–105. https://doi.org/10.1016/j.jddst.2016.06.010.
Shi J, Liu S, Yu Y, He C, Tan L, Shen Y-M. RGD peptide-decorated micelles assembled from polymer–paclitaxel conjugates towards gastric cancer therapy. Colloids Surf B. 2019;180:58–67. https://doi.org/10.1016/j.colsurfb.2019.04.042.
Zhu R, Tian Y. Preparation and evaluation of RGD and TAT co-modified docetaxel-loaded liposome. Drug Des, Dev Ther. 2017;11:3481–9. https://doi.org/10.2147/dddt.S149620.
Wang L, Zhang T, Huo M, Guo J, Chen Y, Xu H. Construction of Nucleus-Targeting Iridium Nanocrystals for Photonic Hyperthermia-Synergized Cancer Radiotherapy. Small. 2019;15(47):1903254. https://doi.org/10.1002/smll.201903254.
Liu W, Lin Q, Fu Y, Huang S, Guo C, Li L, Wang L, Zhang Z, Zhang L. Target delivering paclitaxel by ferritin heavy chain nanocages for glioma treatment. J Control Release. 2020;323:191–202. https://doi.org/10.1016/j.jconrel.2019.12.010.
Mei D, Gong L, Zou Y, Yang D, Liu H, Liang Y, Sun N, Zhao L, Zhang Q, Lin Z. Platelet membrane-cloaked paclitaxel-nanocrystals augment postoperative chemotherapeutical efficacy. J Control Release. 2020;324:341–53. https://doi.org/10.1016/j.jconrel.2020.05.016.
Bang KH, Na YG, Huh HW, Hwang SJ, Kim MS, Kim M, et al. The Delivery Strategy of Paclitaxel Nanostructured Lipid Carrier Coated with Platelet Membrane. Cancers (Basel). 2019;11(6). https://doi.org/10.3390/cancers11060807.
Chai Z, Ran D, Lu L, Zhan C, Ruan H, Hu X, Xie C, Jiang K, Li J, Zhou J, Wang J, Zhang Y, Fang RH, Zhang L, Lu W. Ligand-Modified cell membrane enables the targeted delivery of drug nanocrystals to glioma. ACS Nano. 2019;13(5):5591–601. https://doi.org/10.1021/acsnano.9b00661.
Jiang X, Wang KK, Zhou ZG, Zhang YF, Sha HZ, Xu QP, Wu J, Wang J, Wu J, Hu Y, Liu B. Erythrocyte membrane nanoparticles improve the intestinal absorption of paclitaxel. Biochem Biophys Res Commun. 2017;488(2):322–8. https://doi.org/10.1016/j.bbrc.2017.05.042.
Chen H, Sha HZ, Zhang LR, Qian HQ, Chen FJ, Ding NQ, Ji L, Zhu A, Xu Q, Meng F, Yu L, Zhou Y, Liu B. Lipid insertion enables targeted functionalization of paclitaxel-loaded erythrocyte membrane nanosystem by tumor-penetrating bispecific recombinant protein. Int J Nanomed. 2018;13:5347–59. https://doi.org/10.2147/ijn.S165109.
Wang S, Low PS. Folate-mediated targeting of antineoplastic drags, imaging agents, and nucleic acids to cancer cells. J Control Release. 1998;53(1-3):39–48. https://doi.org/10.1016/s0168-3659(97)00236-8.
Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338(2):284–93. https://doi.org/10.1016/j.ab.2004.12.026.
Chen X, Zhu Q, Xu X, Shen S, Zhang Y, Mo R. Sequentially site-specific delivery of apoptotic protein and tumor-suppressor gene for combination cancer therapy. Small. 2019;15(40). https://doi.org/10.1002/smll.201902998.
Zhang X, Liang N, Gong X, Kawashima Y, Cui F, Sun S. Tumor-targeting micelles based on folic acid and alpha-tocopherol succinate conjugated hyaluronic acid for paclitaxel delivery. Colloids Surf B. 2019;177:11–8. https://doi.org/10.1016/j.colsurfb.2019.01.044.
Narmani A, Mohammadnejad J, Yavari K. Synthesis and evaluation of polyethylene glycol- and folic acid-conjugated polyamidoamine G4 dendrimer as nanocarrier. J Drug Deliv Sci Technol. 2019;50:278–86. https://doi.org/10.1016/j.jddst.2019.01.037.
Qin YT, Peng H, He XW, Li WY, Zhang YK. pH-Responsive Polymer-Stabilized ZIF-8 Nanocomposites for Fluorescence and Magnetic Resonance Dual-Modal Imaging-Guided Chemo-/Photodynamic Combinational Cancer Therapy. ACS Appl Mater Interfaces. 2019;11(37):34268–81. https://doi.org/10.1021/acsami.9b12641.
Pan L, Zhao Y, Yuan Z, Qin G. Research advances on structure and biological functions of integrins. Springerplus. 2016;5:1094. https://doi.org/10.1186/s40064-016-2502-0.
Cheng Y, Ji YH. RGD-modified polymer and liposome nanovehicles: Recent research progress for drug delivery in cancer therapeutics. Eur J Pharm Sci. 2019;128:8–17. https://doi.org/10.1016/j.ejps.2018.11.023.
Daniels TR, Delgado T, Helguera G, Penichet ML. The transferrin receptor part II: Targeted delivery of therapeutic agents into cancer cells. Clin Immunol. 2006;121(2):159–76. https://doi.org/10.1016/j.clim.2006.06.006.
Li HY, Qian ZM. Transferrin/transferrinreceptor-mediated drug delivery. Med Res Rev. 2002;22(3):225–50. https://doi.org/10.1002/med.10008.
Ji B, Maeda A, Higuchi M, Inoue K, Akita H, Harashima H, et al. Pharmacokinetics and brain uptake of lactoferrin in rats. Life Sci. 2006;78(8):851–5. https://doi.org/10.1016/j.lfs.2005.05.085.
Wang Y, Wang Y, Chen G, Li Y, Xu W, Gong S. Quantum-Dot-Based Theranostic Micelles Conjugated with an Anti-EGFR Nanobody for Triple-Negative Breast Cancer Therapy. ACS Appl Mater Interfaces. 2017;9(36):30297–305. https://doi.org/10.1021/acsami.7b05654.
Aswathy J, Seethalekshmy NV, Hiran KR, Bindhu MR, Manzoor K, Nair SV, Menon D. Mn-doped zinc sulphide nanocrystals for immunofluorescent labeling of epidermal growth factor receptors on cells and clinical tumor tissues. Nanotechnol. 2014;25(44):445102. https://doi.org/10.1088/0957-4484/25/44/445102.
Baselga J. Why the epidermal growth factor receptor? The rationale for cancer therapy. Oncologist. 2002;7:2–8.
Nguyen PV, Allard-Vannier E, Chourpa I, Herve-Aubert K. Nanomedicines functionalized with anti-EGFR ligands for active targeting in cancer therapy: Biological strategy, design and quality control. Int J Pharm. 2021;605:120795. https://doi.org/10.1016/j.ijpharm.2021.120795.
Rothenberg ML, Carbone DR, Johnson DH. Improving the evaluation of new cancer treatments: challenges and opportunities. Nat Rev Cancer. 2003;3(4):303–9. https://doi.org/10.1038/nrc1047.
Romero Garcia S. Sullivan Lopez Gonzalez J, Luis Baez Viveros J, Aguilar Cazares D, Prado Garcia H. Tumor cell metabolism An integral view. Cancer Biol Ther. 2011;12(11):939–48. https://doi.org/10.4161/cbt.12.11.18140.
Ji TJ, Zhao Y, Ding YP, Nie GJ. Using Functional Nanomaterials to Target and Regulate the Tumor Microenvironment: Diagnostic and Therapeutic Applications. Adv Mater. 2013;25(26):3508–25. https://doi.org/10.1002/adma.201300299.
Oshi MA, Lee J, Naeem M, Hasan N, Kim J, Kim HJ, Lee EH, Jung Y, Yoo JW. Curcumin Nanocrystal/pH-Responsive Polyelectrolyte Multilayer Core-Shell Nanoparticles for Inflammation-Targeted Alleviation of Ulcerative Colitis. Biomacromolecules. 2020;21(9):3571–81. https://doi.org/10.1021/acs.biomac.0c00589.
Yang J, Gao F, Han D, Yang L, Kong X, Wei M, Cao J, Liu H, Wu Z, Pan G. Multifunctional zinc-based hollow nanoplatforms as a smart pH-responsive drug delivery system to enhance in vivo tumor-inhibition efficacy. Mater Des. 2018;139:172–80. https://doi.org/10.1016/j.matdes.2017.11.004.
Cai X, Luo Y, Zhang W, Du D, Lin Y. pH-Sensitive ZnO quantum dots-doxorubicin nanoparticles for lung cancer targeted drug Delivery. ACS Appl Mater Interfaces. 2016;8(34):22442–50. https://doi.org/10.1021/acsami.6b04933.
Jiang Z, Wang Y, Sun L, Yuan B, Tian Y, Xiang L, Li Y, Li Y, Li J, Wu A. Dual ATP and pH responsive ZIF-90 nanosystem with favorable biocompatibility and facile post-modification improves therapeutic outcomes of triple negative breast cancer in vivo. Biomater. 2019;197:41–50. https://doi.org/10.1016/j.biomaterials.2019.01.001.
Liang P, Zhang W, Kang T, Wu N, Quan C, Zhang C. Design of pH-sensitive supramolecular assembly for cell targeting and controlled release. J Control Release. 2015;213:e17. https://doi.org/10.1016/j.jconrel.2015.05.024.
Zou Y, Yang W, Meng F, Zhong Z. Targeted hepatoma chemotherapy in vivo using galactose-decorated crosslinked pH-sensitive degradable micelles. J Control Release. 2015;213:e125–e6. https://doi.org/10.1016/j.jconrel.2015.05.212.
Zhang JM, Chen RE, Chen FQ, Chen MW, Wang YT. Nucleolin targeting AS1411 aptamer modified pH-sensitive micelles: A dual-functional strategy for paclitaxel delivery. J Control Release. 2015;213:E137–E8. https://doi.org/10.1016/j.jconrel.2015.05.232.
Min KH, Kim JH, Bae SM, Shin H, Kim MS, Park S, Lee H, Park RW, Kim IS, Kim K, Kwon IC, Jeong SY, Lee DS. Tumoral acidic pH-responsive MPEG-poly(beta-amino ester) polymeric micelles for cancer targeting therapy. J Control Release. 2010;144(2):259–66. https://doi.org/10.1016/j.jconrel.2010.02.024.
Xie J, Zhang Y, Yan C, Song L, Wen S, Zang F, Chen G, Ding Q, Yan C, Gu N. High-performance PEGylated Mn-Zn ferrite nanocrystals as a passive-targeted agent for magnetically induced cancer theranostics. Biomater. 2014;35(33):9126–36. https://doi.org/10.1016/j.biomaterials.2014.07.019.
Liu Y, Li M, Yang F, Gu N. Magnetic drug delivery systems. Science China-Materials. 2017;60(6):471–86. https://doi.org/10.1007/s40843-017-9049-0.
Han L, Zhang X-Y, Wang Y-L, Li X, Yang X-H, Huang M, Hu K, Li LH, Wei Y. Redox-responsive theranostic nanoplatforms based on inorganic nanomaterials. J Control Release. 2017;259:40–52. https://doi.org/10.1016/j.jconrel.2017.03.018.
Hu H, Yuan W, Liu F-S, Cheng G, Xu F-J, Ma J. Redox-Responsive Polycation-Functionalized Cotton Cellulose Nanocrystals for Effective Cancer Treatment. ACS Appl Mater Interfaces. 2015;7(16):8942–51. https://doi.org/10.1021/acsami.5b02432.
Xie J, Lee S, Chen XY. Nanoparticle-based theranostic agents. Adv Drug Del Rev. 2010;62(11):1064–79. https://doi.org/10.1016/j.addr.2010.07.009.
Wang J, Hu Y, Chen J, Ye C. Self-assembledCeVO4/Au heterojunction nanocrystals for photothermal/photoacoustic bimodal imaging-guided phototherapy. RSC Adv. 2020;10(5):2581–8. https://doi.org/10.1039/c9ra09860g.
Wang S, Mao J, Liu H, Huang S, Cai J, Gui W, Wu J, Xu J, Shen J, Wang Z. pH-Sensitive nanotheranostics for dual-modality imaging guided nanoenzyme catalysis therapy and phototherapy. J Mater Chem B. 2020;8(22):4859–69. https://doi.org/10.1039/c9tb02731a.
Zhu HB, Li YX, Qiu RQ, Shi L, Wu WT, Zhou SQ. Responsive fluorescent Bi2O3@PVA hybrid nanogels for temperature-sensing, dual-modal imaging, and drug delivery. Biomater. 2012;33(10):3058–69. https://doi.org/10.1016/j.biomaterials.2012.01.003.
Tuomela A, Liu P, Puranen J, Ronkko S, Laaksonen T, Kalesnykas G, et al. Brinzolamide nanocrystal formulations for ophthalmic delivery: reduction of elevated intraocular pressure in vivo. Int J Pharm. 2014;467(1-2):34–41. https://doi.org/10.1016/j.ijpharm.2014.03.048.
Tuomela A, Hirvonen J, Peltonen L. Stabilizing Agents for Drug Nanocrystals: Effect on Bioavailability. Pharmaceutics. 2016;8(2):18. https://doi.org/10.3390/pharmaceutics8020016.
Sun W, Tian W, Zhang Y, He J, Mao S, Fang L. Effect of novel stabilizers-cationic polymers on the particle size and physical stability of poorly soluble drug nanocrystals. Nanomed-Nanotechnol Biol Med. 2012;8(4):460–7. https://doi.org/10.1016/j.nano.2011.07.006.
Kabanov AV, Batrakova EV, Alakhov VY. Pluronic((R)) block copolymers for overcoming drug resistance in cancer. Adv Drug Del Rev. 2002;54(5):759–79. https://doi.org/10.1016/s0169-409x(02)00047-9.
Liu HZ, Ma Y, Liu D, Fallon JK, Liu F. The Effect of Surfactant on Paclitaxel Nanocrystals: An In Vitro and In Vivo Study. J Biomed Nanotechnol. 2016;12(1):147–53. https://doi.org/10.1166/jbn.2016.2127.
Wang S, Wang H, Liu Z, Wang L, Wang X, Su L, Chang J. Smart pH- and reduction-dual-responsive folate-PEG-coated polymeric lipid vesicles for tumor-triggered targeted drug delivery. Nanoscale. 2014;6(13):7635–42. https://doi.org/10.1039/c4nr00843j.
Kanamala M, Palmer BD, Wilson WR, Wu ZM. Characterization of a smart pH-cleavable PEG polymer towards the development of dual pH-sensitive liposomes. Int J Pharm. 2018;548(1):288–96. https://doi.org/10.1016/j.ijpharm.2018.07.009.
Suma T, Miyata K, Anraku Y, Watanabe S, Christie RJ, Takemoto H, Shioyama M, Gouda N, Ishii T, Nishiyama N, Kataoka K. Smart Multilayered Assembly for Biocompatible siRNA Delivery Featuring Dissolvable Silica, Endosome-Disrupting Polycation, and Detachable PEG. Acs Nano. 2012;6(8):6693–705. https://doi.org/10.1021/nn301164a.
Li H, Miteva M, Kirkbride KC, Cheng MJ, Nelson CE, Simpson EM, Gupta MK, Duvall CL, Giorgio TD. Dual MMP7-Proximity-Activated and Folate Receptor-Targeted Nanoparticles for siRNA Delivery. Biomacromolecules. 2015;16(1):192–201. https://doi.org/10.1021/bm501394m.
Razzazan A, Atyabi F, Kazemi B, Dinarvand R. Influence of PEG Molecular Weight on the Drug Release and In vitro Cytotoxicity of Single-Walled Carbon Nanotubes-PEG-Gemcitabine Conjugates. Curr Drug Del. 2016;13(8):1313–24. https://doi.org/10.2174/1567201813666160111123947.
Du XJ, Wang JL, Liu WW, Yang JX, Sun CY, Sun R, et al. Regulating the surface poly(ethylene glycol) density of polymeric nanoparticles and evaluating its role in drug delivery in vivo. Biomater. 2015;69:1–11. https://doi.org/10.1016/j.biomaterials.2015.07.048.
Wu B, Zhang LJ, Zhang CJ, Deng K, Ao YW, Mei H, Zhou W, Wang CX, Yu H, Huang SW. Effect of Poly(ethylene glycol) (PEG) Surface Density on the Fate and Antitumor Efficacy of Redox-Sensitive Hybrid Nanoparticles. ACS Biomater Sci Eng. 2020;6(7):3975–83. https://doi.org/10.1021/acsbiomaterials.0c00516.
Marruecos DF, Kastantin M, Schwartz DK, Kaar JL. Dense Poly(ethylene glycol) Brushes Reduce Adsorption and Stabilize the Unfolded Conformation of Fibronectin. Biomacromolecules. 2016;17(3):1017–25. https://doi.org/10.1021/acs.biomac.5b01657.
Hou WM, Miyazaki S, Takada M, Komai T. Sustained release of indomethacin from chitosan granules. Chem Pharm Bull (Tokyo). 1985;33(9):3986–92.
Miyazaki S, Ishii K, Nadai T. The use of chitin and chitosan as drug carriers. Chem Pharm Bull (Tokyo). 1981;29(10):3067–9.
Miyazaki S, Yamaguchi H, Yokouchi C, Takada M, Hou WM. Sustained release of indomethacin from chitosan granules in beagle dogs. J Pharm Pharmacol. 1988;40(9):642–3. https://doi.org/10.1111/j.2042-7158.1988.tb05325.x.
Quan P, Shi K, Piao H, Piao H, Liang N, Xia D, Cui F. A novel surface modified nitrendipine nanocrystals with enhancement of bioavailability and stability. Int J Pharm. 2012;430(1-2):366–71. https://doi.org/10.1016/j.ijpharm.2012.04.025.
Moghaddam AS, Khonakdar HA, Sarikhani E, Jafari SH, Javadi A, Shamsi M, Amirkhani MA, Ahadian S. Fabrication of Carboxymethyl Chitosan Nanoparticles to Deliver Paclitaxel for Melanoma Treatment. ChemNanoMat. 2020;6(9):1373–85. https://doi.org/10.1002/cnma.202000229.
Paiva D, Ivanova G. Pereira MdC, Rocha S. Chitosan conjugates for DNA delivery. Phys Chem Chem Phys. 2013;15(28):11893–9. https://doi.org/10.1039/c3cp51215k.
Sahariah P, Masson M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure-Activity Relationship. Biomacromolecules. 2017;18(11):3846–68. https://doi.org/10.1021/acs.biomac.7b01058.
Pandey N, Soto-Garcia LF, Liao J, Zimmern P, Nguyen KT, Hong Y. Mussel-inspired bioadhesives in healthcare: design parameters, current trends, and future perspectives. Biomater Sci. 2020;8(5):1240–55. https://doi.org/10.1039/c9bm01848d.
Jiang J, Zhu L, Zhu L, Zhu B, Xu Y. Surface Characteristics of a Self-Polymerized Dopamine Coating Deposited on Hydrophobic Polymer Films. Langmuir. 2011;27(23):14180–7. https://doi.org/10.1021/la202877k.
Kang SM, Hwang NS, Yeom J, Park SY, Messersmith PB, Choi IS, Langer R, Anderson DG, Lee H. One-Step Multipurpose Surface Functionalization by Adhesive Catecholamine. Adv Funct Mater. 2012;22(14):2949–55. https://doi.org/10.1002/adfm.201200177.
Zhan H, Jagtiani T, Liang JF. A new targeted delivery approach by functionalizing drug nanocrystals through polydopamine coating. Eur J Pharm Biopharm. 2017;114:221–9. https://doi.org/10.1016/j.ejpb.2017.01.020.
Hao YN, Zheng AQ, Guo TT, Shu Y, Wang JH, Johnson O, Chen W. Glutathione triggered degradation of polydopamine to facilitate controlled drug release for synergic combinational cancer treatment. J Mater Chem B. 2019;7(43):6742–50. https://doi.org/10.1039/c9tb01400d.
Yurkin ST, Wang Z. Cell membrane-derived nanoparticles: emerging clinical opportunities for targeted drug delivery. Nanomedicine. 2017;12(16):2007–19. https://doi.org/10.2217/nnm-2017-0100.
Li RX, He YW, Zhang SY, Qin J, Wang JX. Cell membrane-based nanoparticles: a new biomimetic platform for tumor diagnosis and treatment. Acta Pharm Sin B. 2018;8(1):14–22. https://doi.org/10.1016/j.apsb.2017.11.009.
Su J, Sun H, Meng Q, Yin Q, Tang S, Zhang P, Chen Y, Zhang Z, Yu H, Li Y. Long Circulation Red-Blood-Cell-Mimetic Nanoparticles with Peptide-Enhanced Tumor Penetration for Simultaneously Inhibiting Growth and Lung Metastasis of Breast Cancer. Adv Funct Mater. 2016;26(8):1243–52. https://doi.org/10.1002/adfm.201504780.
Rao L, Meng QF, Bu LL, Cai B, Huang Q, Sun ZJ, Zhang WF, Li A, Guo SS, Liu W, Wang TH, Zhao XZ. Erythrocyte Membrane-Coated Upconversion Nanoparticles with Minimal Protein Adsorption for Enhanced Tumor Imaging. ACS Appl Mater Interfaces. 2017;9(3):2159–68. https://doi.org/10.1021/acsami.6b14450.
Zhu DM, Xie W, Xiao YS, Suo M, Zan MH, Liao QQ, Hu XJ, Chen LB, Chen B, Wu WT, Ji LW, Huang HM, Guo SS, Zhao XZ, Liu QY, Liu W. Erythrocyte membrane-coated gold nanocages for targeted photothermal and chemical cancer therapy. Nanotechnol. 2018;29(8):084002. https://doi.org/10.1088/1361-6528/aa9ca1.
Funding
This study was supported by National Natural Science Foundation of China (No. 22078234).
Author information
Authors and Affiliations
Contributions
Conceptualization: Meng Bai, Zhenping Wei; Formal analysis: Meng Bai; Writing - Review & Editing: Meng Bai; Supervision: Zhenping Wei, Mingshi Yang, Junbo Gong; Project administration: Zhenping Wei; Funding acquisition: Junbo Gong. All authors contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bai, M., Yang, M., Gong, J. et al. Progress and Principle of Drug Nanocrystals for Tumor Targeted Delivery. AAPS PharmSciTech 23, 41 (2022). https://doi.org/10.1208/s12249-021-02200-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02200-w