Abstract
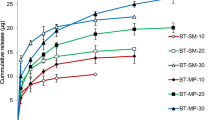
Currently, glaucoma is managed by frequent instillation of bimatoprost eye drop therapy, which showed very poor ocular bioavailability. Contact lens is widely used as medical device to improve the drug retention on the ocular tissues. However, the traditional methods of drug loading in the contact lens matrix showed high burst release and changes the optophysical properties of the contact lens material. In this paper, a novel bimatoprost-loaded silica shell nanoparticles-laden soft contact lenses were developed to achieve sustain drug delivery without altering the optophysical properties of the contact lens. Silica-shell nanoparticles were prepared using octyltrimethoxysilane (OTMS) and microemulsion. Traditional soaking method (SM-BT), direct bimatoprost loading method (DL-BT), and microemulsion-laden contact lens (ME-BT) were developed for comparison. The silica shell-coated nanoparticles-laden soft contact lenses (SiS-BT) showed improved swelling, transmittance, oxygen permeability, and lysozyme adherence compared to SM-BT, DL-BT, and ME-BT lenses. The DL-BT and ME-BT batch showed high bimatoprost lost/leaching during extraction and sterilization steps, with low cumulative drug release. Also, SiS-BT lens showed sustain bimatoprost release for 96 h. In a rabbit tear fluid model, the SiS-BT lens showed high bimatoprost concentration for 72 h compared to ME-BT lens and eye drop therapy. Based on histopathological studies of cornea, the SiS-BT lens was found to be safe for human applications. The data demonstrated the novel application of silica shell nanoparticles to deliver bimatoprost from the contact lens for extended period of time without altering the optophysical properties of the contact lens.





Similar content being viewed by others
Data Availability
The raw data are available on request from the corresponding author.
Change history
31 May 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1208/s12249-022-02306-9
References
Susanna R, De Moraes CG, Cioffi GA, Ritch R. Why do people (still) go blind from glaucoma? Transl Vision Sci Technol. 2015;4(2):1–10.
Pan Y, Varma R. Natural history of glaucoma. Indian J Ophthalmol. 2011;59(Suppl1):S19.
van der Valk R, Webers CA, Schouten JS, Zeegers MP, Hendrikse F, Prins MH. Intraocular pressure–lowering effects of all commonly used glaucoma drugs: a meta-analysis of randomized clinical trials. Ophthalmology. 2005;112(7):1177–85.
Brasnu E, Brignole-Baudouin F, Riancho L, Guenoun J-M, Warnet J-M, Baudouin C. In vitro effects of preservative-free tafluprost and preserved latanoprost, travoprost, and bimatoprost in a conjunctival epithelial cell line. Curr Eye Res. 2008;33(4):303–12.
Maulvi FA, Desai DT, Shetty KH, Shah DO, Willcox MD. Advances and challenges in the nanoparticles-laden contact lenses for ocular drug delivery. Int J Pharm. 2021;608:121090.
Desai A, Shukla M, Maulvi F, Ranch K. Ophthalmic and otic drug administration: novel approaches and challenges. In: Misra A, Shahiwala A, editors. Novel drug delivery technologies. Singapore: Springer; 2019. pp. 335–81.
Goldberg I, Pina RG, Lanzagorta-Aresti A, Schiffman RM, Liu C, Bejanian M. Bimatoprost 0.03%/timolol 0.5% preservative-free ophthalmic solution versus bimatoprost 0.03%/timolol 0.5% ophthalmic solution (Ganfort) for glaucoma or ocular hypertension: A 12-week randomised controlled trial. Br J Ophthalmol. 2014;98(7):926–31.
Harasymowycz P, Hutnik C, Rouland J-F, Negrete FJM, Economou MA, Denis P, Baudouin C. Preserved versus preservative-free latanoprost for the treatment of glaucoma and ocular hypertension: a post hoc pooled analysis. Adv Ther. 2021:1–13.
Stringham J, Ashkenazy N, Galor A, Wellik SR. Barriers to glaucoma medication compliance among veterans: Dry eye symptoms and anxiety disorders. Eye & Contact Lens. 2018;44(1):50–60.
Choi SW, Kim J. Therapeutic contact lenses with polymeric vehicles for ocular drug delivery: A review. Mater Sci Eng C. 2018;11(7):1125.
Maulvi FA, Soni TG, Shah DO. A review on therapeutic contact lenses for ocular drug delivery. Drug Deliv. 2016;23(8):3017–26.
Soluri A, Hui A, Jones L. Delivery of ketotifen fumarate by commercial contact lens materials. Optom Vis Sci. 2012;89(8):1140–9.
Torres-Luna C, Hu N, Tammareddy T, Domszy R, Yang J, Wang NS, Yang A. Extended delivery of non-steroidal anti-inflammatory drugs through contact lenses loaded with Vitamin E and cationic surfactants. Contact Lens Anterior Eye. 2019;42(5):546–52.
Zhu Y, Sheng Y. Sustained delivery of epalrestat to the retina using PEGylated solid lipid nanoparticles laden contact lens. Int J Pharm. 2020;587:119688.
Ribeiro A, Veiga F, Santos D, Torres-Labandeira JJ, Concheiro A, Alvarez-Lorenzo C. Hydrophilic acrylic hydrogels with built-in or pendant cyclodextrins for delivery of anti-glaucoma drugs. Carbohydr Polym. 2012;88(3):977–85.
Abeer MM, Rewatkar P, Qu Z, Talekar M, Kleitz F, Schmid R, Lindén M, Kumeria T, Popat A. Silica nanoparticles: A promising platform for enhanced oral delivery of macromolecules. J Control Release. 2020;326:544–55.
Meka AK, Jenkins LJ, Dàvalos-Salas M, Pujara N, Wong KY, Kumeria T, Mariadason JM, Popat A. Enhanced solubility, permeability and anticancer activity of vorinostat using tailored mesoporous silica nanoparticles. Pharmaceutics. 2018;10(4):283–90.
Abbaraju PL, Meka AK, Jambhrunkar S, Zhang J, Xu C, Popat A, Yu C. Floating tablets from mesoporous silica nanoparticles. J Mater Chem B. 2014;2(47):8298–302.
Abeer MM, Meka AK, Pujara N, Kumeria T, Strounina E, Nunes R, Costa A, Sarmento B, Hasnain SZ, Ross BP. Rationally designed dendritic silica nanoparticles for oral delivery of exenatide. Pharmaceutics. 2019;11(8):418.
Yan F, Liu Y, Han S, Zhao Q, Liu N. Bimatoprost imprinted silicone contact lens to treat glaucoma. AAPS PharmSciTech. 2020;21(2):1–8.
Karlgard C, Wong N, Jones L, Moresoli C. In vitro uptake and release studies of ocular pharmaceutical agents by silicon-containing and p-HEMA hydrogel contact lens materials. Int J Pharm. 2003;257(1-2):141–51.
Sekar P, Chauhan A. Effect of vitamin-E integration on delivery of prostaglandin analogs from therapeutic lenses. J Colloid Interface Sci. 2019;539:457–67.
Koli AR, Ranch KM, Patel HP, Parikh RK, Shah DO, Maulvi FA. Oral bioavailability improvement of felodipine using tailored microemulsion: Surface science, ex vivo and in vivo studies. Int J Pharm. 2021;596:120202.
Sriramulu D, Reed EL, Annamalai M, Venkatesan TV, Valiyaveettil S. Synthesis and characterization of superhydrophobic, self-cleaning NIR-reflective silica nanoparticles. Sci Rep. 2016;6(1):1–10.
Venkatathri N, Yoo J. Synthesis and characterization of silica nanosphere from octadecyltrimethoxy silane. Bull Kor Chem Soc. 2008;29(1):29–30.
Maulvi FA, Mangukiya MA, Patel PA, Vaidya RJ, Koli AR, Ranch KM, Shah DO. Extended release of ketotifen from silica shell nanoparticle-laden hydrogel contact lenses: in vitro and in vivo evaluation. J Mater Sci Mater Med. 2016;27(6):113.
Pandey SS, Patel MA, Desai DT, Patel HP, Gupta AR, Joshi SV, Shah DO, Maulvi FA. Bioavailability enhancement of repaglinide from transdermally applied nanostructured lipid carrier gel: optimization, in vitro and in vivo studies. J Drug Deliv Sci Technol. 2020;57:101731.
Maulvi FA, Patel PJ, Soni PD, Desai AR, Desai DT, Shukla MR, Ranch KM, Shah SA, Shah DO. Novel poly (vinylpyrrolidone)-coated silicone contact lenses to improve tear volume during lens wear: in vitro and in vivo studies. ACS Omega. 2020;5(29):18148–54.
Alvarez-Rivera F, Concheiro A, Alvarez-Lorenzo C. Epalrestat-loaded silicone hydrogels as contact lenses to address diabetic-eye complications. Eur J Pharm Biopharm. 2018;122:126–36.
Gulsen D, Chauhan A. Effect of water content on transparency, swelling, lidocaine diffusion in p-HEMA gels. J Membr Sci. 2006;269(1-2):35–48.
Phan C-M, Weber S, Mueller J, Yee A, Jones L. A rapid extraction method to quantify drug uptake in contact lenses. Transl Vis Sci Technol. 2018;7(2):11.
Lee SE, Kim SR, Park M. Oxygen permeability of soft contact lenses in different pH, osmolality and buffering solution. Intern J Ophthalmol. 2015;8(5):1037.
Tran N-P-D, Yang M-C. The ophthalmic performance of hydrogel contact lenses loaded with silicone nanoparticles. Polymers. 2020;12(5):1128.
Luensmann D, Jones L. Albumin adsorption to contact lens materials: a review. Contact Lens Anterior Eye. 2008;31(4):179–87.
Senchyna M, Jones L, Louie D, May C, Forbes I, Glasier M-A. Quantitative and conformational characterization of lysozyme deposited on balafilcon and etafilcon contact lens materials. Curr Eye Res. 2004;28(1):25–36.
Subbaraman LN, Glasier M-A, Senchyna M, Jones L. Stabilization of lysozyme mass extracted from lotrafilcon silicone hydrogel contact lenses. Optom Vis Sci. 2005;82(3):209–14.
Ciolino JB, Hoare TR, Iwata NG, Behlau I, Dohlman CH, Langer R, Kohane DS. A drug-eluting contact lens. Invest Ophthalmol Vis Sci. 2009;50(7):3346–52.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1208/s12249-022-02306-9
Supplementary Information
ESM 1
(DOCX 44 kb)
About this article
Cite this article
Xiaojie, H., Fagang, J., Jun, J. et al. RETRACTED ARTICLE: Bimatoprost-Loaded Silica Shell–Coated Nanoparticles-Laden Soft Contact Lenses to Manage Glaucoma: In Vitro and In Vivo Studies. AAPS PharmSciTech 23, 33 (2022). https://doi.org/10.1208/s12249-021-02199-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02199-0