Abstract
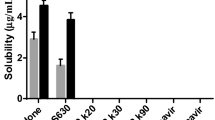
The focus of the present investigation was to develop amorphous glassy solutions (AGSs) of BCS Class II and IV drugs using sucrose acetate isobutyrate (SAIB). The drugs studied were rifaximin (RFX), dasatinib (DST), aripiprazole (APZ), dolutegravir (DLT), cyclosporine (CYS), itraconazole (ITZ), tacrolimus (TAC), sirolimus (SRL), aprepitant (APT), and carbamazepine (CBZ). AGSs were prepared by dissolving known quantity of the drug in the SAIB at 120 (TAC and APZ), 140 (CYS) or 150 oC (RFX, DST, DLT, ITZ, SRL, APT, and CBZ). They were characterized visually and by NIR, NIR hyperspectroscopy (NIR-H), and XRPD. Stability were determined by exposing open vials to 40 oC/75% RH for a week. AGSs behave like a glassy solid at room temperature and liquified above 60 oC. The solubility of APT, DLT, SRL, APZ, RFX, CBZ, TAC and CYS in SAIB was 0.4±0.0, 1.7±0.4, 1.9±0.0, 21.6±2.6, 36.4±0.9, 76.5±4.0, 115.1±2.3, and 239.0±12.6 mg/g, respectively. NIR, NIR-H, and XRPD data indicated the amorphous nature of the AGSs. Furthermore, AGSs were stable against devitrification on exposure to high temperature and humidity. In summary, SAIB can be employed to develop stable AGSs of poorly soluble drugs to increase dissolution, and oral bioavailability with the addition of hydrophilic excipients.










Similar content being viewed by others
References
FDA guidance for industry - Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system, 2017.
Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutics drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20.
Yu LX, Amidon GL, Polli JE, Zhao H, Mehta MU, Conner DP, Shah VP, Lesko LJ, Chen ML, Lee VHL, Hussain AS. Biopharmaceutics classification system: The scientific basis for biowaiver extensions. Pharm Res. 2002;19:921–5.
Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5:442–53.
Van den Mooter G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today. 2012;9:e71–e174.
Reichardt C. Solvents and Solvent Effects in Organic Chemistry. 3rd ed. Weinheim, Germany: Wiley–VCH; 2004.
Rahman Z, Zidan AS, Samy R, Sayeed VA, Khan MA. Improvement of physicochemical properties of an antiepileptic drug by salt engineering. AAPS PharmSciTech. 2012;13:793–801.
Hauel N, Narr B, Ries U, Van Meel JC, Wienen W, Entzeroth M, inventors; Karl Thomae GmbH, assignee. Benzimidazoles useful as angiotensin-11 antagonists. United States Patent US 5591762. 1997.
Rahman Z, Siddiqui A, Khan MA. Assessing the impact of nimodipine devitrification in the ternary cosolvent system through quality by design approach. Int J Pharm. 2013;455:113–23.
Taxotere FDA label. Accessed on June 05, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020449s084lbl.pdf
Rahman Z, Siddiqui A, Bykadi S, Khan MA. Determination of tacrolimus crystalline fraction in the commercial immediate release amorphous solid dispersion products by a standardized X-ray powder diffraction method with chemometrics. Int J Pharm. 2014;475:462–70.
Laitinen R, Löbmann K, Strachan CJ, Grohganz H, Rades T. Emerging trends in the stabilization of amorphous drugs. Int J Pharm. 2013;453:65–79.
Rahman Z, Zidan AS, Khan MA. Risperidone solid dispersion for orally disintegrating tablet: its formulation design and non-destructive methods of evaluation. Int J Pharm. 2010;400:49–58.
Loftsson T, Duchêne D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329:1–11.
List M, Sucker H. Pharmaceutical colloidal hydrosols for injection. GB Patent 2200048. 1998.
Baker M, Naguib M. Propofol: the challenges of formulation. Anesthesiol. 2005;103:860–76.
Skrdla PJ, Floyd PD, Dell'Orco PC. Predicting the solubility enhancement of amorphous drugs and related phenomena using basic thermodynamic principles and semi-empirical kinetic models. Int J Pharm. 2019;567:118465.
Peltonen L, Strachan CJ. Degrees of Order: A Comparison of nanocrystal and amorphous solids for poorly soluble drugs. Int J Pharm. 2020;586:119492.
Jha DK, Shah DS, Amin PD. Thermodynamic aspects of the preparation of amorphous solid dispersions of naringenin with enhanced dissolution rate. Int J Pharm. 2020;583:119363.
Schver GCRM, Nadvorny D, Lee PI. Evolution of supersaturation from amorphous solid dispersions in water-insoluble polymer carriers: effects of swelling capacity and interplay between partition and diffusion. Int J Pharm. 2020;581:119292.
Chen B, Wang X, Zhang Y, Huang K, Liu H, Xu D, Li S, Liu Q, Huang J, Yao H, Lin X. Improved solubility, dissolution rate, and oral bioavailability of main biflavonoids from Selaginella doederleinii extract by amorphous solid dispersion. Drug Deliv. 2020;27:309–22.
Sironi D, Bauer-Brandl A, Brandl M, Rosenberg J, Fricker G. The influence of liquid intake on the performance of an amorphous solid dispersion in rats. Eur J Pharm Biopharm. 2020;152:296–8.
Jermain SV, Miller D, Spangenberg A, Lu X, Moon C, Su Y, Williams RO. Homogeneity of amorphous solid dispersions - an example with KinetiSol(®). Drug Dev Ind Pharm. 2019;45:724–35.
Abreu-Villela R, Schönenberger M, Caraballo I, Kuentz M. Early stages of drug crystallization from amorphous solid dispersion via fractal analysis based on chemical imaging. Eur J Pharm Biopharm. 2018;133:122–30.
Alonzo DE, Zhang GZG, Zhou D, Gao Y, Taylor LS. Understanding the behavior of amorphous pharmaceutical systems during dissolution. Pharm Res. 2010;27:608–18.
Gao P, Shi Y. Characterization of supersaturatable formulations for improved absorption of poorly soluble drugs. AAPS J. 2012;14:703–13.
Dinunzio JC, Miller DA, Yang W, Mcginity JW, Williams RO 3rd. Amorphous compositions using concentration enhancing polymers for improved bioavailability of itraconazole. Mol. Pharm. 2008;5:968–80.
Miller DA, Dinunzio JC, Yang W, Mcginity JW, Williams RO 3rd. Enhanced in vivo absorption of itraconazole via stabilization of supersaturation following acidic-to-neutral ph transition. Drug Dev Ind Pharm. 2008;34:890–902.
Sharma PK, Panda A, Pradhan A, Zhang J, Thakkar R, Whang CH, Repka MA, Murthy SN. Solid-state stability issues of drugs in transdermal patch formulations. AAPS. 2008;19:27–35.
European Medicines Agency. European Medicines Agency agrees to precautionary recall of Advagraf 0.5 mg capsule batches 2011. Accessed on Nov 30, 2020. https://www.ema.europa.eu/en/news/european-medicines-agency-agrees-precautionary-recall-advagraf-05-mg-capsule-batches.
Wilson V, Lou X, Osterling DJ, Stolarik DF, Jenkins G, Gao W, Zhang GGZ, Taylor LS. Relationship between amorphous solid dispersion in vivo absorption and in vitro dissolution: phase behavior during dissolution, speciation, and membrane mass transport. Journal of Controlled Release. 2018;292:172–82.
Purohit HS, Trasi NS, Osterling DJ, Stolarik DF, Jenkins GJ, Gao W, Zhang GGZ, Taylor LS. Assessing the Impact of Endogenously Derived Crystalline Drug on the in Vivo Performance of Amorphous Formulations. Mol Pharm. 2019 Aug 5;16(8):3617–25.
EFSA Panel on Food additives and Nutrient Sources added to Food (ANS). Re-evaluation of sucrose acetate isobutyrate (E 444) as a food additive. EFSA J. 2016;14(5):4489.
SAIB safety data sheet. Accessed on May 27, 2021. http://ws.eastman.com/ProductCatalogApps/PageControllers/MSDS_PC.aspx?Product=71001070
Code of Federal Regulations Title 21 - Sucrose acetate isobutyrate, Section 172.833, Part 172, Food additive permitted for direct addition to food for human consumption. Accessed on May 27, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.833
CFSAN/Office of Food Additive Safety-Agency Response Letter GRAS Notice No. GRN 000623, August 1, 2016. Accessed on Dec 16, 2021. https://www.fda.gov/food/gras-notice-inventory/agency-response-letter-gras-notice-nogrn-000623
Tant MA. Biopharmaceutic and pharmacokinetic studies of sucrose acetate isobutyrate as an excipient for oral drug delivery. Electronic Theses and Dissertations.2011, Paper 1345. Accessed on Sept 14, 2021.https://dc.etsu.edu/etd/1345.
Lu Y, Yu Y, Tang X. Sucrose acetate isobutyrate as an in situ forming system for sustained risperidone release. J Pharm Sci. 2007 Dec;96(12):3252–62.
Dharani S, Barakh Ali SF, Afrooz H, Mohamed EM, Cook P, Khan MA, Rahman Z. Development of methamphetamine abuse-deterrent formulations using sucrose acetate isobutyrate. J Pharm Sci. 2020 Mar;109(3):1338–46.
Barakh Ali SF, Dharani S, Afrooz H, Mohamed EM, Cook P, Khan MA, Rahman Z. Development of abuse-deterrent formulations using sucrose acetate isobutyrate. AAPS PharmSciTech. 2020;21(3):99.
Harloff-Helleberg S, Fliervoet LAL, Fanø M, Schmitt M, Antopolski M, Urtti A, Nielsen HM. Exploring the mucoadhesive behavior of sucrose acetate isobutyrate: a novel excipient for oral delivery of biopharmaceuticals. Drug Deliv. 2019 Dec;26(1):532–41.
Posimir FDA label. Accessed on Sept 14. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/204803s000lbl.pdf
SucroMateTM Equine FDA label. Accessed on Sept 14. https://animaldrugsatfda.fda.gov/adafda/views/#/home/previewsearch/141-319
ICH – Validation of analytical procedures: Text and methodology, Q2(R1), 2005.
Dharani S, Rahman Z, Sogra FBA, Hamideh A, Khan MA. Quantitative estimation of phenytoin sodium disproportionation in the formulations using vibration spectroscopies and multivariate methodologies. Int J Pharm. 2018;539:65–74.
PubChem-National Library of Medicine. Accessed on Sept 14, 2021. https://pubchem.ncbi.nlm.nih.gov/
Li Y, Pang H, Guo Z, Lin L, Dong Y, Li G, Lu M, Wu C. Interactions between drugs and polymers influencing hot melt extrusion. J Pharm Pharmacol. 2014;66(2):148–66.
Jankovic S, Tsakiridou G, Ditzinger F, Koehl NJ, Price DJ, Ilie AR, Kalantzi L, Kimpe K, Holm R, Nair A, Griffin B, Saal C, Kuentz M. Application of the solubility parameter concept to assist with oral delivery of poorly water-soluble drugs - a PEARRL review. J Pharm Pharmacol. 2019;71(4):441–63.
Salahinejad M, Le TC, Winkler DA. Capturing the crystal: Prediction of enthalpy of sublimation, crystal lattice energy, and melting points of organic compounds. J. Chem. Inf. Model. 2013;53:223–9.
Ong HJ, Pinal R. Drug Solubilization by means of a surface-modified edible biopolymer enabled by hot melt extrusion. J Pharm Sci. 2018;107(1):402–11.
De la Guardia M, Armenta S. Avoiding Sample Treatments. In: Comprehensive analytical chemistry. Elsevier; 2011. p. 59–86.
Scotter CNG. Infrared spectroscopy. In: Encyclopedia of analytical science by Worsfold P, Townshend A, Poole C. Elsevier; 2005. p. 415–26.
Rahman Z, Siddiqui A, Bykadi S, Khan MA. Near-infrared and fourier transform infrared chemometric methods for the quantification of crystalline tacrolimus from sustained-release amorphous solid dispersion. J Pharm Sci. 2014;103:2376–85.
Korang-Yeboah M, Akhtar S, Siddiqui A, Rahman Z, Khan MA. Application of NIR chemometric methods for quantification of the crystalline fraction of warfarin sodium in drug product. Drug Dev Ind Pharm. 2016;42:584–94.
Zidan AS, Rahman Z, Sayeed V, Raw A, Yu L, Khan MA. Crystallinity evaluation of tacrolimus solid dispersions by chemometric analysis. Int J Pharm. 2012;423:341–50.
Berthiaux H, Mosorov V, Tomczak L, Gatumel C, Demeyre JF. Principal component analysis for characterising homogeneity in powder mixing using image processing techniques. Chem. Eng Process. 2006;45:397–403.
Khuroo T, Dharani S, Mohamed EM, Immadi S, Wu Z, Khan MA, Lu D, Nehete P, Rahman Z. Ultra-long acting prodrug of dolutegravir and delivery system - Physicochemical, pharmacokinetic and formulation characterizations. Int J Pharm. 2021;607:120889.
Zou F, Chen Q, Yang P, Zhou J, Wu J, Zhuang W, Ying H. Solution-Mediated Polymorphic Transformation: From Amorphous to Crystals of Disodium Guanosine 5′-Monophosphate in Ethanol. Ind Eng Chem Res. 2017;56:8274–82.
Tian B, Ding Z, Zong S, Yang J, Wang N, Wang T, Huang X, Hao H. Manipulation of pharmaceutical polymorphic transformation process using excipients. Curr Pharm Des. 2020;26(21):2553–63.
Frank DS, Matzger AJ. Effect of polymer hydrophobicity on the stability of amorphous solid dispersions and supersaturated solutions of a hydrophobic pharmaceutical. Mol Pharm. 2019;16(2):682–8.
Ilevbare GA, Liu H, Edgar KJ, Taylor LS. Maintaining supersaturation in aqueous drug solutions: Impact of different polymers on induction times. Cryst. Growth Des. 2013;13(2):740–51.
Johnson LM, Li Z, LaBelle AJ, Bates FS, Lodge TP, Hillmyer MA. Impact of polymer excipient molar mass and end groups on hydrophobic drug solubility enhancement. Macromolecules. 2017;50(3):1102–12.
Mosquera-Giraldo LI, Borca CH, Meng X, Edgar KJ, Slipchenko LV, Taylor LS. Mechanistic design of chemically diverse polymers with applications in oral drug delivery. Biomacromolecules. 2016;17(11):3659–71.
Dharani S, Barakh Ali SF, Afrooz H, Mohamed EM, Cook P, Khan MA, Rahman Z. Development of methamphetamine abuse-deterrent formulations using sucrose acetate isobutyrate. Journal of Pharmaceutical Sciences. 2020;109(3):1338–46.
Funding
The research reported in this paper was funded by Eastman Chemical Company, Kingsport, TN.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dharani, S., Sediri, K., Cook, P. et al. Preparation and Characterization of Stable Amorphous Glassy Solution of BCS II and IV Drugs. AAPS PharmSciTech 23, 35 (2022). https://doi.org/10.1208/s12249-021-02198-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02198-1