Abstract
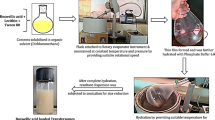
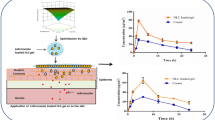
The in vitro dissolution of Avanafil (AVA) is the rate-limiting step for its bioavailability. Also, it undergoes the first-pass metabolism, and its absorption is altered significantly in the presence of food. So, our study aimed to overcome the previous hurdles and improve the AVA bioavailability by its incorporation in the ultra-deformable nanovesicles, transfersomes (TRF), then loading these nanovesicles in transdermal films. The AVA-loaded TRF formulation was optimized using Draper-Lin small composite design (D-LSCD). The optimized AVA-loaded TRF was evaluated for quality attributes and assessed for skin permeation using a fluorescence laser microscope and for pharmacokinetic parameters after topical application on the rats. The optimized AVA-loaded TRF showed a vesicle size of 97.75 nm, a zeta potential of −28.83 mV, and entrapment efficiency of 95.14% with good deformability and release profile. The intense discoloration in the deep skin layers of the rats indicated the permeation efficiency of AVA-loaded TRF films. The pharmacokinetic parameters specified the augmented absorption extent with Cmax of 254.66 ± 8.02 ng/mlversus 70.33 ± 3.05 ng/ml which reflected on the AUC0-inf that has a value of 2050.45 ± 159.14 ng/ml h versus 497.34 ± 102.61 ng/ml h for the optimized AVA-loaded TRF film and raw AVA-loaded film, respectively. These promising results wide open the field for broader clinical application of this alternative delivery pathway for superior bioavailability, efficacy, and patient compliance and satisfaction.
Graphical abstract








Similar content being viewed by others
References
Jumani DK. New approach to management of erectile dysfunction in 2017. Beyond Med. 2017:1044–50.
Hackett G, Kirby M, Wylie K, Heald A, Ossei-Gerning N, Edwards D, Muneer A. British Society for Sexual Medicine guidelines on the management of erectile dysfunction in men—2017. J Sex Med. Elsevier Inc. 2018;15:430–57.
El-Sakka AI. Erectile dysfunction in Arab countries. Part I: Prevalence and correlates. Arab J Urol Arab Assoc of Urology. 2012;10:97–103.
Fahmy UA. Nanoethosomal transdermal delivery of vardenafil for treatment of erectile dysfunction: Optimization, characterization, and in vivo evaluation. Drug Des Devel Ther. 2015;9:6129–37.
Corona G, Maggi M, Jannini E. Avanafil: The second-generation treatment of erectile dysfunction. Eur Med J. 2016:61–9.
Saxena A, Parakash P, Porwal M, Sissodia N, Sharma P. Erectile dysfunction: A review and herbs used for its treatment. Int J Green Pharm. 2012;6:109–17.
Burke RM, Evans JD. Avanafil for treatment of erectile dysfunction: Review of its potential. Vasc Health Risk Manag. 2012;8:517–23.
Patel A, Nair A, Prajapati P, Jadhav A. Formulation and evaluation of Avanafil orodispersible tablet. Int J Chem Pharm Sci. 2015;3:1975–86.
Hosny KM, Ahmed OAA, Fahmy UA, Alkhalidi HM. Nanovesicular systems loaded with a recently approved second generation type-5 phospodiesterase inhibitor (avanafil): I. Plackett-Burman screening and characterization. J Drug Deliv Sci Technol. 2018;43:154–9.
Shrestha H, Bala R, Arora S. Lipid-based drug delivery systems. Aust J Pharm. 2014;2014:10.
Cevc G, Blume G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. BBA - Biomembr. 1992;1104:226–32.
Ahmed TA. Preparation of transfersomes encapsulating sildenafil aimed for transdermal drug delivery: Plackett–Burman design and characterization. J Liposome Res [Internet]. 2015;25:1–10. https://doi.org/10.3109/08982104.2014.950276.
Pawar AY, Jadhav KR, Chaudhari LH. Transfersome: A novel technique which improves transdermal permeability. Asian J Pharm. 2016;10:425–36.
Kumar A, Pathak K, Bali V. Ultra-adaptable nanovesicular systems: A carrier for systemic delivery of therapeutic agents. Drug Discov Today [Internet]. Elsevier Ltd; 2012 [cited 2014 Apr 19];17:1233–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22766375
Pradhan M, Singh D, Singh MR. Novel colloidal carriers for psoriasis: Current issues, mechanistic insight and novel delivery approaches. J Control release [Internet]. 2013 [cited 2014 Feb 26];170:380–95. Available from: http://www.sciencedirect.com/science/article/pii/S0168365913003106
Sarmah PJ, Kalita B, Sharma AK. Transfersomes based transdermal drug delivery: An overview. Int J Adv Pharm Res. 2013;4:2555–63.
Sciences M, Sarkar BK, Maharshi A, Baniwal A, Kumar S, Road B, et al. Formulation and characterization of quercetin transfersome for transdermal delivery. Int J Pharm Med Sci. 2012;1:28–38.
Al Shuwaili AH, Rasool BKA, Abdulrasool AA. Optimization of elastic transfersomes formulations for transdermal delivery of pentoxifylline. Eur J Pharm Biopharm. Elsevier B.V. 2016;102:101–14.
Patel R, Singh SK, Singh S, Sheth NR, Gendle R. Development and characterization of curcumin loaded transfersome for transdermal delivery. J Pharm Sci Res. 2009;1:71–80.
El-Say KM, Hosny KM. Optimization of carvedilol solid lipid nanoparticles: An approach to control the release and enhance the oral bioavailability on rabbits. PLoS ONE. 2018;13(8):e0203405. https://doi.org/10.1371/journal.pone.0203405.
Fahmy UAAB. Stability indicating HPLC method for analysis of avanafil using diode array detector. Int J Adv Pharm Biol Chem. 2016;5:59–64.
Ahmed TA, El-Say KM, Aljaeid BM, Fahmy UA, Abd-Allah FI. Transdermal glimepiride delivery system based on optimized ethosomal nano-vesicles: Preparation, characterization, in vitro, ex vivo and clinical evaluation. Int Joumal Pharm [Internet]. Elsevier B.V.; 2016;500:245–54. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0378517316300187
El-Say KM, Ahmed TA, Badr-Eldin SM, Fahmy U, Aldawsari H, Ahmed OAA. Enhanced permeation parameters of optimized nanostructured simvastatin transdermal films: Ex vivo and in vivo evaluation. Pharm Dev Technol [Internet]. 2015 [cited 2014 Oct 20];20:919–26. https://doi.org/10.3109/10837450.2014.938859.
Ahmed TA, El-Say KM. Development of alginate-reinforced chitosan nanoparticles utilizing W/O nanoemulsification/internal crosslinking technique for transdermal delivery of rabeprazole. Life Sci [Internet]. Elsevier Inc.; 2014 [cited 2014 Oct 13];110:35–43. : http://www.ncbi.nlm.nih.gov/pubmed/24997393
Basahih TS, Alamoudi AA, El-Say KM, Alhakamy NA, Ahmed OAA. Improved transmucosal delivery of glimepiride via unidirectional release buccal film loaded with vitamin E TPGS-based nanocarrier. Dose-Response. 2020;18:1–12.
Alamoudi AA, Ahmed OAA, El-Say KM. Investigating the potential of transdermal delivery of Avanafil using vitamin E-TPGS based mixed micelles loaded films. Pharmaceutics. 2021;13:739.
Ahmed OAA, El-Say KM, Aljaeid BM, Badr-Eldin SM, Ahmed TA. Optimized vinpocetine-loaded vitamin E D-α-tocopherol polyethylene glycol 1000 succinate-alpha lipoic acid micelles as a potential transdermal drug delivery system: In vitro and ex vivo studies. Int J Nanomedicine. 2018;14:33–43.
Thavarajah R, Mudimbaimannar VK, Elizabeth J, Rao UK, Ranganathan K. Chemical and physical basics of routine formaldehyde fixation. J Oral Maxillofac Pathol. 2012;16:400–5.
El-Say KM, El-Helw A, Ahmed OAA, Hosny M, Ahmed TA, Kharshoum RM, et al. Statistical optimization of controlled release microspheres containing cetirizine hydrochloride as a model for water soluble drugs. Pharm Dev Technol. 2015;20.
Jamal M, Imam SS, Aqil M, Amir M, Mir SR, Mujeeb M. Transdermal potential and anti-arthritic efficacy of ursolic acid from niosomal gel systems. Int Immunopharmacol. Elsevier B.V. 2015;29:361–9.
Pardakhty A, Varshosaz J, Rouholamini A. In vitro study of polyoxyethylene alkyl ether niosomes for delivery of insulin. Int J Pharm [Internet]. 2007 [cited 2014 Dec 17];328:130–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16997517
Das S, Ng WK, Tan RBH. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur J Pharm Sci [Internet]. Elsevier B.V. 2012;47:139–51. https://doi.org/10.1016/j.ejps.2012.05.010.
Smith MC, Crist RM, Clogston JD, McNeil SE. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal Bioanal Chem Anal Bioanal Chem. 2017;409:5779–87.
Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M, Ali A. Formulation and optimization of nanotransfersomes using experimental design technique for accentuated transdermal delivery of valsartan. Nanomedicine Nanotechnology. Biol Med. Elsevier Inc. 2012;8:237–49.
El Zaafarany GM, Awad GAS, Holayel SM, Mortada ND. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int J Pharm [Internet]. Elsevier B.V.; 2010 [cited 2014 Apr 23];397:164–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20599487
Bayindir ZS, Yuksel N. Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J Pharm Sci [Internet]. 2010 [cited 2014 Dec 11];99:2049–60. Available from: . http://www.ncbi.nlm.nih.gov/pubmed/19780133
Lin HW, Xie QC, Huang X, Ban JF, Wang B, Wei X, Chen Y, Lu Z. Increased skin permeation efficiency of imperatorin via charged ultradeformable lipid vesicles for transdermal delivery. Int J Nanomedicine. 2018;13:831–42.
Song CK, Balakrishnan P, Shim C-KK, Chung S-JJ, Chong S, Kim D-DD. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: Characterization and in vitro/in vivo evaluation. Colloids Surf B Biointerfaces [Internet]. Elsevier B.V. 2012;92:299–304. https://doi.org/10.1016/j.colsurfb.2011.12.004.
Dave V, Yadav RB, Ahuja R, Yadav S. Formulation design and optimization of novel fast dissolving tablet of chlorpheniramine maleate by using lyophilization techniques. Bull Fac Pharmacy, Cairo Univ Faculty of Pharmacy, Cairo University. 2017;55:31–9.
El-Laithy HM, Shoukry O, Mahran LG. Novel sugar esters proniosomes for transdermal delivery of vinpocetine: preclinical and clinical studies. Eur J Pharm Biopharm [Internet]. Elsevier B.V.; 2011 [cited 2014 Apr 23];77:43–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21056658
Nasr M, Mansour S, Mortada ND, Elshamy AA. Vesicular aceclofenac systems: a comparative study between liposomes and niosomes. J Microencapsul [Internet]. Informa UK Ltd UK. 2008;25:499–512. https://doi.org/10.1080/02652040802055411.
Johnston MJW, Semple SC, Klimuk SK, Edwards K, Eisenhardt ML, Leng EC, Karlsson G, Yanko D, Cullis PR. Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations. Biochim Biophys Acta. 1758;2006:55–64.
Yehia R, Hathout RM, Attia DA, Elmazar MM, Mortada ND. Anti-tumor efficacy of an integrated methyl dihydrojasmonate transdermal microemulsion system targeting breast cancer cells: In vitro and in vivo studies. Colloids Surf B Biointerfaces [Internet]. Elsevier B.V. 2017;155:512–21. https://doi.org/10.1016/j.colsurfb.2017.04.031.
Ahmed OAA, Badr-Eldin SM. In situ misemgel as a multifunctional dual-absorption platform for nasal delivery of raloxifene hydrochloride: Formulation, characterization, and in vivo performance. Int J Nanomedicine [Internet]. 2018;13:6325–35 https://www.dovepress.com/in-situ-misemgel-as-a-multifunctional-dual-absorption-platform-for-nas-peer-reviewed-article-IJN.
Acknowledgements
The authors acknowledge with thanks to DSR for technical and financial support.
Funding
This research was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (G:351-166-1441).
Author information
Authors and Affiliations
Contributions
Conceptualization, A.A. and K.E.; methodology, O.D.A., A.A., and O.A.A.; software, K.E.; validation, A.A. and K.E.; formal analysis, K.E.; investigation, O.D.A., A.A., and O.A.A.; resources, O.D.A. and K.E.; data curation, O.D.A., A.A., O.A.A., and K.E.; writing—original draft preparation, O.D.A. and K.E.; writing—review and editing, O.D.A., A.A., O.A.A., and K.E.; visualization, O.D.A. and K.E.; supervision, A.A., O.A., and K.E.; project administration, K.E.; funding acquisition, K.E. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Research Ethics Committee, Faculty of Pharmacy, King Abdulaziz University (Approval No. PH-119-41).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-hejaili, O.D., Alamoudi, A.A., Ahmed, O.A.A. et al. Transdermal Film Loaded with Avanafil Ultra-deformable Nanovesicles to Enhance its Percutaneous Absorption and Bioavailability. AAPS PharmSciTech 23, 46 (2022). https://doi.org/10.1208/s12249-021-02195-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02195-4