Abstract
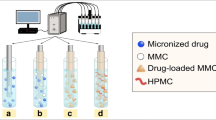
The objective of the study is to develop a quick and simultaneous analysis system for the dissolution of the active pharmaceutical ingredient (API) and the formulation excipient in samples from the dissolution test by UHPLC using the charged aerosol and PDA detectors. The combination of two columns for size-exclusion chromatography (SEC) and the equipment of the charged aerosol detector allowed the quick determination of various water-soluble polymers. Three model sustained-release tablets, each containing a different API of different water solubility (propranolol (soluble), ranitidine (very soluble), and cilostazol (practically insoluble)), were prepared from polyethylene oxide (PEO) matrix to verify the applicability and utility of the analysis system. The dissolution of propranolol was the same as that of PEO, indicating that the diffusion rate of propranolol was consistent with the erosion rate of the PEO and that the dissolution of PRO was based on diffusion. Ranitidine was released faster than PEO, suggesting that ranitidine was diffused through the gel layer of PEO early upon contact with the dissolution medium and before PEO gel erosion. Cilostazol was released slower as compared to PEO, indicating that cilostazol dissolution was based on the polymer’s erosion. These results suggested that the analysis system developed in this study is a precise and valid tool to study the dissolution behavior of both APIs and excipients. Optimization of the SEC column for the appropriate separation of APIs and excipients makes the analysis system more efficient and convenient to study the drug release mechanisms and to design formulations.





Similar content being viewed by others
References
Kadajji VG, Betageri GV. Water soluble polymers for pharmaceutical applications. Polymers. 2011;3:1972–2009.
Tran P, Pyo YC, Kim DH, Lee SE, Kim JK, Park JS. Overview of the manufacturing methods of solid dispersion technology for improving the solubility of poorly water-soluble drugs and application to anticancer drugs. Pharmaceutics. 2019;11:132–57.
Debotton N, Dahan A. Applications of polymers as pharmaceutical excipients in solid oral dosage forms. Med Res Rev. 2017;37:52–97.
Mašková E, Kubová K, Raimi-Abraham BT, Vllasaliu D, Vohlídalová E, Turánek J, et al. Hypromellose – a traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J Control Release. 2020;324:695–727.
Dahl TC, Calderwood T, Bormeth A, Trimble K, Piepmeier E. Influence of physico-chemical properties of hydroxypropyl methylcellulose on naproxen release from sustained release matrix tablets. J Control Release. 1990;14:1–10.
Alvarez-Lorenzo C, Castro E, Gómez-Amoza JL, Martínez-Pacheco R, Souto C, Concheiro A. Intersupplier and interlot variability in hydroxypropyl celluloses: implications for theophylline release from matrix tablets. Pharm Acta Helv. 1998;73:113–20.
Vanhoorne V, Janssens L, Vercruysse J, De Beer T, Remon JP, Vervaet C. Continuous twin screw granulation of controlled release formulations with various HPMC grades. Int J Pharm. 2016;511:1048–57.
Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. Understanding pharmaceutical quality by design. AAPS J. 2014;16:771–83.
ICH Q8 (R2): Pharmaceutical development. International Conference on Harmonization, Geneva; 2009.
FDA Guidance for industry: immediate release solid oral dosage forms scale-up and postapproval changes: Chemistry, manufacturing, and controls, in vitro dissolution testing, and in vivo bioequivalence documentation.
FDA Guidance for industry: SUPAC MR: Modified release solid oral dosage forms: scale-up and post-approval changes: chemistry, manufacturing and controls, in vitro dissolution testing, and in vivo bioequivalence.
FDA Guidance for industry: waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system.
Kataoka M, Sugano K, da Costa MC, Wong JW, Jones KL, Masaoka Y, et al. Application of dissolution/permeation system for evaluation of formulation effect on oral absorption of poorly water-soluble drugs in drug development. Pharm Res. 2012;29:1485–94.
Miyaji Y, Fujii Y, Takeyama S, Kawai Y, Kataoka M, Takahashi M, et al. Advantage of the dissolution/permeation system for estimating oral absorption of drug candidates in the drug discovery stage. Mol Pharm. 2016;13:1564–74.
Borbás E, Nagy ZK, Nagy B, Balogh A, Farkas B, Tsinman O, et al. The effect of formulation additives on in vitro dissolution-absorption profile and in vivo bioavailability of telmisartan from brand and generic formulations. Eur J Pharm Sci. 2018;114:310–7.
Miller-Chou BA, Koenig JK. A review of polymer dissolution. Prog Polym Sci. 2003;28:1223–70.
Shojaee S, Nokhodchi A, Maniruzzaman M. Evaluation of the drug solubility and rush ageing on drug release performance of various model drugs from the modified release polyethylene oxide matrix tablets. Drug Deliv and Transl Res. 2017;7:111–24.
Ghori MU, Ginting G, Smith AM, Conway BR. Simultaneous quantification of drug release and erosion from hypromellose hydrophilic matrices. Int J Pharm. 2014;465:405–12.
Kojima H, Yoshihara K, Sawada T, Kondo H, Sako K. Extended release of a large amount of highly water-soluble diltiazem hydrochloride by utilizing counter polymer in polyethylene oxides (PEO)/polyethylene glycol (PEG) matrix tablets. Eur J Pharm Biopharm. 2008;70:556–62.
Chaerunisaa AY, Ali R, Dashevskiy A. Release adjustment of two drugs with different solubility combined in a matrix tablet. AAPS PharmSciTech. 2019;20:142.
Ohara T, Kitamura S, Kitagawa T, Terada K. Dissolution mechanism of poorly water-soluble drug from extended release solid dispersion system with ethyl cellulose and hydroxypropyl-methylcellulose. Int J Pharm. 2005;302:95–102.
Meinel S, Germershaus L, Holzgrabe OU. Simple and rapid high performance liquid chromatography method for the determination of polidocanol as bulk product and in pharmaceutical polymer matrices using charged aerosol detection. J Pharm Biomed Anal. 2015;104:17–20.
Ligor M, Studzińska S, Horna A, Buszewski B. Corona-charged aerosol detection: an analytical approach. Crit Rev Anal Chem. 2013;43:64–78.
Kou D, Manius G, Zhan S, Chokshi HP. Size exclusion chromatography with Corona charged aerosol detector for the analysis of polyethylene glycol polymer. J Chromatogr A. 2009;1216:5424–8.
Shojaee S, Emami P, Mahmood A, Rowaiye Y, Dukulay A, Kaialy W, et al. An investigation on the effect of polyethylene oxide concentration and particle size in modulating theophylline release from tablet matrices. AAPS PharmSciTech. 2015;16:1281–9.
Kim CJ. Drug release from compressed hydrophilic Polyox® WSR tablets. J Pharm Sci. 1995;84:303–6.
Kim CJ. Effects of drug solubility, drug loading and polymer molecular weight on drug release from Polyox® tablets. Drug Dev Ind Pharm. 1998;24:645–51.
ICH. Guideline Q2. Validation of analytical procedures: text and methodology R1. International Conference on Harmonization, Geneva; 2005.
Jain AK, Söderlind E, Viridén A, Schug B, Abrahamsson B, Knopke C, et al. The influence of hydroxypropyl methylcellulose (HPMC) molecular weight, concentration and effect of food on in vivo erosion behavior of HPMC matrix tablets. J Control Release. 2014;187:50–8.
FDA Guidance for industry: Dissolution Testing of Immediate Release Solid Oral Dosage Forms.
Colombo P, Bettini R, Santi P, Peppas NA. Swellable matrices for controlled drug delivery: gel-layer behaviour, mechanisms and optimal performance. Pharm Sci Technol Today. 2000;3:198–204.
Acknowledgements
We would like to thank Mr. Nishibayashi, Mr. Jinno, Mr. Kiba, and Mr. Yoshimura for giving us the opportunity to do this research and Mr. Mizunaga for technical advice.
Funding
The PEO-4NF and PEO-8NF used in this study is a product under development by Sumitomo Seika Industries, Ltd., and was provided as a sample.
Author information
Authors and Affiliations
Contributions
Substantial contribution to the conception or design of the work, M. Kimoto; Contribution to the acquisition, analysis, or interpretation of the data for the work, M. Kimoto; Drafting the work, M. Kimoto; Revising it critically for important intellectual content, T. Sakane; Final approval of the version to be published, T. Sakane, H. Katsumi, A. Yamamoto.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kimoto, M., Sakane, T., Katsumi, H. et al. Quick and Simultaneous Analysis of Dissolved Active Pharmaceutical Ingredients and Formulation Excipients from the Dissolution Test Utilizing UHPLC and Charged Aerosol Detector. AAPS PharmSciTech 22, 262 (2021). https://doi.org/10.1208/s12249-021-02152-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02152-1