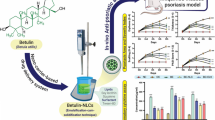
Abstract
Lipid-polymer hybrid nanoparticles display several benefits over either lipid and/or polymer based systems with respect to enhanced drug loading, good colloidal stability, sustained release profile, and high cellular uptake. The present work rivets on development and evaluation of vitamin D3-loaded monolithic lipid-polymer hybrid nanoparticles (VD3/LPHNPs) for their in vivo anti-psoriatic efficacy. These LPHNPs were prepared using a hot homogenization method and exhibited spherical morphology with a lower particle size (123.1 nm) with narrow PDI (0.234) and efficient encapsulation (76.80%). Further, these LPHNPs demonstrated a sustained release profile of VD3 for up to 3 days following a Korsemeyer-Peppas release model. Further, VD3/LPHNPs were formulated into a topical gel containing 0.005% w/w of VD3. Rheological data suggested that the product exhibited non-newtonian flow properties with characteristic shear-thinning and variable thixotropy features that are desirable for topical formulation. The successful formation of gel structure and its long-term stability were confirmed from the oscillatory studies such as amplitude and frequency sweep tests. In vivo efficacy assessment in imiquimod-induced psoriatic mouse model demonstrated enhanced anti-psoriatic activity of VD3 with improved PASI score when delivered as LPHNPs gel as compared to the free VD3 gel that were further supported by histopathology and immunohistochemistry.
Graphic Abstract











Similar content being viewed by others
References
Dainichi T, Kitoh A, Otsuka A, Nakajima S, Nomura T, Kaplan DH, et al. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat Immunol. 2018;19(12):1286–98.
Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445(7130):866–73.
Lee YS, Lee M-H, Kim H-J, Won H-R, Kim C-H. Non-thermal atmospheric plasma ameliorates imiquimod-induced psoriasis-like skin inflammation in mice through inhibition of immune responses and up-regulation of PD-L1 expression. Sci Rep. 2017;7(1):1–12.
Gottlieb AB. Psoriasis: emerging therapeutic strategies. Nat Rev Drug Discov. 2005;4(1):19–34.
Sala M, Elaissari A, Fessi H. Advances in psoriasis physiopathology and treatments: up to date of mechanistic insights and perspectives of novel therapies based on innovative skin drug delivery systems (ISDDS). J Control Release. 2016;239:182–202.
Murphy EC, Schaffter SW, Friedman AJ. Nanotechnology for psoriasis therapy. Curr Dermatol Rep. 2019;8(1):14–25.
Lebwohl M, Ting P, Koo J. Psoriasis treatment: traditional therapy. Ann Rheum Dis. 2005;64(suppl 2):ii83–6.
Bhat M, Pukale S, Singh S, Mittal A, Chitkara D. Nano-enabled topical delivery of anti-psoriatic small molecules. J Drug Deliv Sci Technol. 2021;62:102328.
Menter A, Griffiths CE. Current and future management of psoriasis. Lancet. 2007;370(9583):272–84.
Bikle DD, Nemanic MK, Gee E, Elias P. 1, 25-Dihydroxyvitamin D3 production by human keratinocytes Kinetics and regulation. J Clin Invest. 1986;78(2):557–66.
Bikle DD, Nemanic MK, Whitney JO, Elias PW. Neonatal human foreskin keratinocytes produce 1, 25-dihydroxyvitamin D3. Biochemistry. 1986;25(7):1545–8.
Lehmann B. The vitamin D3 pathway in human skin and its role for regulation of biological processes. Photochem Photobiol. 2005;81(6):1246–51.
Lehmann B, Sauter W, Knuschke P, Dressler S, Meurer M. Demonstration of UVB-induced synthesis of 1α, 25-dihydroxyvitamin D 3 (calcitriol) in human skin by microdialysis. Arch Dermatol Res. 2003;295(1):24–8.
Bikle DD. Vitamin D and the skin: physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13(1):3–19.
Popadic S, Ramic Z, Medenica L, Stojkovic MM, Trajković V, Popadic D. Antiproliferative effect of vitamin A and D analogues on adult human keratinocytes in vitro. Skin Pharmacol Physiol. 2008;21(4):227–34.
Chen TC, Persons KS, Lu Z, Mathieu JS, Holick MF. An evaluation of the biologic activity and vitamin D receptor binding affinity of the photoisomers of vitamin D3 and previtamin D3. J Nutr Biochem. 2000;11(5):267–72.
Ingram MA, Jones MB, Stonehouse W, Jarrett P, Scragg R, Mugridge O, et al. Oral vitamin D3 supplementation for chronic plaque psoriasis: a randomized, double-blind, placebo-controlled trial. J Dermatolog Treat. 2018;29(7):648–57.
Lalloz A, Bolzinger M-A, Faivre J, Latreille P-L, Ac AG, Rakotovao C, et al. Effect of surface chemistry of polymeric nanoparticles on cutaneous penetration of cholecalciferol. Int J Pharm. 2018;553(1–2):120–31.
Ramezanli T, Kilfoyle BE, Zhang Z, Michniak-Kohn BB. Polymeric nanospheres for topical delivery of vitamin D3. Int J Pharm. 2017;516(1–2):196–203.
Pukale SS, Sharma S, Dalela M, Kumar Singh A, Mohanty S, Mittal A, et al. Multi-component clobetasol-loaded monolithic lipid-polymer hybrid nanoparticles ameliorate imiquimod-induced psoriasis-like skin inflammation in Swiss albino mice. Acta Biomater. 2020;115:393–409.
Zhang Z, Tsai PC, Ramezanli T, Michniak-Kohn BB. Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5(3):205–18.
Sakdiset P, Amnuaikit T, Pichayakorn W, Pinsuwan S. Formulation development of ethosomes containing indomethacin for transdermal delivery. J Drug Deliv Sci Technol. 2019;52:760–8.
Sun L, Liu Z, Wang L, Cun D, Tong HH, Yan R, et al. Enhanced topical penetration, system exposure and anti-psoriasis activity of two particle-sized, curcumin-loaded PLGA nanoparticles in hydrogel. J Control Release. 2017;254:44–54.
Şenyiğit T, Sonvico F, Barbieri S, Özer Ö, Santi P, Colombo P. Lecithin/chitosan nanoparticles of clobetasol-17-propionate capable of accumulation in pig skin. J Control Release. 2010;142(3):368–73.
Shah PP, Desai PR, Patel AR, Singh MS. Skin permeating nanogel for the cutaneous co-delivery of two anti-inflammatory drugs. Biomaterials. 2012;33(5):1607–17.
Desai PR, Marepally S, Patel AR, Voshavar C, Chaudhuri A, Singh M. Topical delivery of anti-TNFα siRNA and capsaicin via novel lipid-polymer hybrid nanoparticles efficiently inhibits skin inflammation in vivo. J Control Release. 2013;170(1):51–63.
Desai P, Patlolla RR, Singh M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol Membr Biol. 2010;27(7):247–59.
Kaur A, Katiyar SS, Kushwah V, Jain S. Nanoemulsion loaded gel for topical co-delivery of clobitasol propionate and calcipotriol in psoriasis. Nanomedicine. 2017;13(4):1473–82.
Sonawane R, Harde H, Katariya M, Agrawal S, Jain S. Solid lipid nanoparticles-loaded topical gel containing combination drugs: an approach to offset psoriasis. Expert Opin Drug Deliv. 2014;11(12):1833–47.
Arora R, Katiyar SS, Kushwah V, Jain S. Solid lipid nanoparticles and nanostructured lipid carrier-based nanotherapeutics in treatment of psoriasis: a comparative study. Expert Opin Drug Deliv. 2017;14(2):165–77.
Date T, Nimbalkar V, Kamat J, Mittal A, Mahato RI, Chitkara D. Lipid-polymer hybrid nanocarriers for delivering cancer therapeutics. J Control Release. 2018;271:60–73.
Sharma S, Mazumdar S, Italiya KS, Date T, Mahato RI, Mittal A, et al. Cholesterol and morpholine grafted cationic amphiphilic copolymers for miRNA-34a delivery. Mol Pharm. 2018;15(6):2391–402.
D’Souza S. A review of in vitro drug release test methods for nano-sized dosage forms. Advances in Pharmaceutics. 2014;2014.
Jain AK, Thareja S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif Cells Nanomed Biotechnol. 2019;47(1):524–39.
D’Souza SS, DeLuca PP. Methods to assess in vitro drug release from injectable polymeric particulate systems. Pharm Res. 2006;23(3):460–74.
Weng J, Tong HH, Chow SF. In vitro release study of the polymeric drug nanoparticles: development and validation of a novel method. Pharmaceutics. 2020;12(8):732.
Guichard A, Humbert P, Tissot M, Muret P, Courderot-Masuyer C, Viennet C. Effects of topical corticosteroids on cell proliferation, cell cycle progression and apoptosis: in vitro comparison on HaCaT. Int J Pharm. 2015;479(2):422–9.
Wen D, Chitkara D, Wu H, Danquah M, Patil R, Miller DD, et al. LHRH-conjugated micelles for targeted delivery of antiandrogen to treat advanced prostate cancer. Pharm Res. 2014;31(10):2784–95.
Mazumdar S, Italiya KS, Sharma S, Chitkara D, Mittal A. Effective cellular internalization, cell cycle arrest and improved pharmacokinetics of Tamoxifen by cholesterol based lipopolymeric nanoparticles. Int J Pharm. 2018;543(1–2):96–106.
Kothari IR, Mazumdar S, Sharma S, Italiya K, Mittal A, Chitkara D. Docetaxel and alpha-lipoic acid co-loaded nanoparticles for cancer therapy. Ther Deliv. 2019;10(4):227–40.
Horváth S, Komlódi R, Perkecz A, Pintér E, Gyulai R, Kemény Á. Methodological refinement of Aldara-induced psoriasiform dermatitis model in mice. Sci Rep. 2019;9(1):1–8.
Kim JY, Ahn J, Kim J, Choi M, Jeon H, Choe K, et al. Nanoparticle-assisted transcutaneous delivery of a signal transducer and activator of transcription 3-inhibiting peptide ameliorates psoriasis-like skin inflammation. ACS Nano. 2018;12(7):6904–16.
Kang N-W, Kim M-H, Sohn S-Y, Kim K-T, Park J-H, Lee S-Y, et al. Curcumin-loaded lipid-hybridized cellulose nanofiber film ameliorates imiquimod-induced psoriasis-like dermatitis in mice. Biomaterials. 2018;182:245–58.
Vasseur P, Pohin M, Jégou J, Favot L, Venisse N, Mcheik J, et al. Liver fibrosis is associated with cutaneous inflammation in the imiquimod-induced murine model of psoriasiform dermatitis. Br J Dermatol. 2018;179(1):101–9.
Hashimoto Y, Tsutsui M, Matsuo S, Iizuka H. Flow cytometric analysis of pig epidermal keratinocytes: effects of ultraviolet B irradiation (UVB) and topical PUVA treatment. J Dermatol Sci. 1995;10(1):16–24.
Boehncke W-H. Topical photodynamic therapy for psoriasis. Comprehensive Series in Photosciences: Elsevier; 2001. p. 259–70.
Witman PM, editor. Topical therapies for localized psoriasis. Mayo Clin Proc. 2001;76:943–949.
Mezei M, Gulasekharam V. Liposomes-a selective drug delivery system for the topical route of administration I Lotion dosage form. Life Sci. 1980;26(18):1473–7.
Alam MS, Ali MS, Alam N, Siddiqui MR, Shamim M, Safhi M. In vivo study of clobetasol propionate loaded nanoemulsion for topical application in psoriasis and atopic dermatitis. Drug Invent. Today. 2013;5(1):8–12.
Aggarwal G, Nagpal M, Kaur G. Development and comparison of nanosponge and niosome based gel for the topical delivery of tazarotene. Pharm Nanotechnol. 2016;4(3):213–28.
Jin Y, Zhang X, Zhang B, Kang H, Du L, Li M. Nanostructures of an amphiphilic zinc phthalocyanine polymer conjugate for photodynamic therapy of psoriasis. Colloids Surf B Biointerfaces. 2015;128:405–9.
Lu GW, Gao P. Emulsions and microemulsions for topical and transdermal drug delivery. Handbook of non-invasive drug delivery systems: Elsevier; 2010. p. 59–94.
Shnoudeh AJ, Hamad I, Abdo RW, Qadumii L, Jaber AY, Surchi HS, et al. Synthesis, characterization, and applications of metal nanoparticles. Biomaterials and bionanotechnology: Elsevier; 2019. p. 527–612.
Shen J, Burgess DJ. In vitro dissolution testing strategies for nanoparticulate drug delivery systems: recent developments and challenges. Drug Deliv Transl Res. 2013;3(5):409–15.
Acknowledgements
The authors acknowledged Incisive Element LLC, USA, for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
This work was funded by Incisive Element LLC, USA, and might result into commercial products that can be licenced to other pharmaceutical companies or developed in house, in which the authors have a commercial and/or financial interest. The authors (DC and AM) are the founding directors of Nanobrid Innovations Private Limited, India, that is involved in the development of nanotechnology-based products. They have a business and/or financial interest in the operations of the company. The same could be disclosed on request.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pukale, S.S., Mittal, A. & Chitkara, D. Topical Application of Vitamin D3-Loaded Hybrid Nanosystem to Offset Imiquimod-Induced Psoriasis. AAPS PharmSciTech 22, 238 (2021). https://doi.org/10.1208/s12249-021-02116-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02116-5