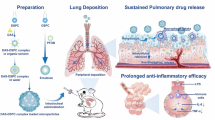
Abstract
Risedronate sodium (RS) is a potent nitrogen-containing bisphosphonate which is known to induce osteoclast apoptosis. As a drug repurposing approach, the current work explored the potential of nebulizable RS-chitosan (CS) microspheres to induce alveolar macrophage apoptosis. RS-CS microspheres were assessed for lung deposition, cytotoxicity, and cellular uptake percentage in Calu-3 cells. The potential of nebulizable microspheres for treating elastase-induced emphysema in rats was investigated, compared to RS marketed oral tablets®, with respect to histopathological, immunohistochemical, and flow cytometric studies. The in vitro lung deposition pattern suggested deep alveolar deposition of RS microspheres, with respect to high FPF% and suitable MMAD (66% and 1.506 μm, respectively, at a flow rate of 28.3 L min−1). No apparent cytotoxicity was observed, with a cell viability > 90%. The inhalation of RS-CS microspheres was suggested to inhibit airspace enlargement and lung rarefaction after elastase instillation and reduce the macrophage accumulation in alveolar parenchyma. Immunohistochemical and cytometric analyses revealed significant low expression levels of CD68 and CD11b surface markers, respectively, with significantly (P < 0.05) lower detected numbers of intact alveolar macrophages following inhalation of RS-CS microspheres. The nebulization of RS-CS microspheres could induce apoptosis in alveolar macrophages and be promisingly adopted for attenuation of pulmonary emphysema.






Similar content being viewed by others
References
The top 10 causes of death [Internet]. [cited 2020 Dec 25]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
Geiser M, Quaile O, Wenk A, Wigge C, Eigeldinger-Berthou S, Hirn S, et al. Cellular uptake and localization of inhaled gold nanoparticles in lungs of mice with chronic obstructive pulmonary disease. Part Fibre Toxicol. 2013;10:1–10.
Seijo LM, Zulueta JJ. Understanding the links between lung cancer, COPD, and emphysema: a key to more effective treatment and screening. Oncology (Williston Park). 2017. p. 93–102.
Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front Immunol Front Res Foundation. 2014;5.
Ueno M, Maeno T, Nishimura S, Ogata F, Masubuchi H, Hara K, et al. Alendronate inhalation ameliorates elastase-induced pulmonary emphysema in mice by induction of apoptosis of alveolar macrophages. Nat Commun. Nature Publishing Group; 2015;6:6332.
Yamasaki K, Van Eeden SF. Lung macrophage phenotypes and functional responses: role in the pathogenesis of COPD. Int J Mol Sci. 2018;19.
Rogers TL, Holen I. Tumour macrophages as potential targets of bisphosphonates. J. Transl. Med. BioMed Central Ltd.; 2011
Cecchini MG, Fleisch H. Bisphosphonates in vitro specifically inhibit, among the hematopoietic series, the development of the mouse mononuclear phagocyte lineage. J Bone Miner Res. 1990;5:1019–27.
Dunford JE, Rogers MJ, Ebetino FH, Phipps RJ, Coxon FP. Inhibition of protein prenylation by bisphosphonates causes sustained activation of Rac, Cdc42, and Rho GTPases. J Bone Miner Res. 2006;21:684–94.
Grenha A, Al-Qadi S, Seijo B, Remuñán-López C. The potential of chitosan for pulmonary drug delivery. J Drug Deliv Sci Technol Elsevier. 2010;20:33–43.
Lee W-H, Loo C-Y, Traini D, Young PM. Nano- and micro-based inhaled drug delivery systems for targeting alveolar macrophages. Expert Opin Drug Deliv Informa Healthcare. 2015;12:1009–26.
Meenach SA, Kim YJ, Kauffman KJ, Kanthamneni N, Bachelder EM, Ainslie KM. Synthesis, optimization, and characterization of camptothecin-loaded acetalated dextran porous microparticles for pulmonary delivery. Mol Pharm. 2012;9:290–8.
Pramanik S, Sali V. Connecting the dots in drug delivery: a tour d’horizon of chitosan-based nanocarriers system. Int J Biol Macromol Elsevier B.V.; 2021. p. 103–21.
Marasini N, Haque S, Kaminskas LM. Polymer-drug conjugates as inhalable drug delivery systems: a review. Curr. Opin. Colloid Interface Sci. Elsevier Ltd; 2017. p. 18–29.
Park J-H, Jin H-E, Kim D-D, Chung S-J, Shim W-S, Shim C-K. Chitosan microspheres as an alveolar macrophage delivery system of ofloxacin via pulmonary inhalation. Int J Pharm Elsevier. 2013;441:562–9.
Bianco ID, Balsinde J, Beltramo DM, Castagna LF, Landa CA, Dennis EA. Chitosan-induced phospholipase A2 activation and arachidonic acid mobilization in P388D1 macrophages. FEBS Lett. 2000;466:292–4.
Shibata Y, Foster LA, Metzger WJ, Myrvik QN. Alveolar macrophage priming by intravenous administration of chitin particles, polymers of N-acetyl-D-glucosamine, in mice. Infect Immun. 1997;65:1734–41.
Elkady OA, Tadros MI, El-laithy HM. QbD approach for novel crosslinker-free ionotropic gelation of risedronate sodium–chitosan nebulizable microspheres: optimization and characterization. AAPS PharmSciTech. 2020;21:14.
Arafa HMM, Abdel-Wahab MH, El-Shafeey MF, Badary OA, Hamada FMA. Anti-fibrotic effect of meloxicam in a murine lung fibrosis model. Eur J Pharmacol Elsevier. 2007;564:181–9.
Courrier HM, Butz N, Vandamme TF. Pulmonary drug delivery systems: recent developments and prospects; Crit Rev Ther Drug Carrier Syst. Begel House Inc.; 2002;19:425–98.
Marple V, Santhanakrishnan K, Mitchell JP, Roberts DL, Hudson-curtis BL. Next generation pharmaceutical impactor : a new. J Aerosol Med. 2004;17:335–43.
Abdelrahim ME. Aerodynamic characteristics of nebulized terbutaline sulphate using the Andersen Cascade Impactor compared to the Next Generation Impactor. Pharm Dev Technol. 2011;16:137–45.
Elbary AA, El-laithy HM, Tadros MI. Promising ternary dry powder inhaler formulations of cromolyn sodium: Formulation and in vitro-in vivo evaluation. Arch Pharm Res Pharmaceutical Society of Korea. 2007;30:785–92.
Morrow BH, Payne GF, Shen JK. Titration properties and pH-dependent aggregation of chitosan. Biophys J Elsevier; 2015;108:488a.
Nasr M, Awad GAS, Mansour S, Taha I, Shamy A Al, Mortada ND. Different modalities of NaCl osmogen in biodegradable microspheres for bone deposition of risedronate sodium by alveolar targeting. Eur J Pharm Biopharm Elsevier; 2011;79:601–611.
Pallagi E, Karimi K, Ambrus R, Szabó-Révész P, Csóka I. New aspects of developing a dry powder inhalation formulation applying the quality-by-design approach. Int J Pharm Elsevier. 2016;511:151–60.
Pellosi DS, d’Angelo I, Maiolino S, Mitidieri E, d’Emmanuele di Villa Bianca R, Sorrentino R, et al. In vitro/in vivo investigation on the potential of Pluronic® mixed micelles for pulmonary drug delivery. Eur J Pharm Biopharm Elsevier; 2018;130:30–38.
Said-Elbahr R, Nasr M, Alhnan MA, Taha I, Sammour O. Nebulizable colloidal nanoparticles co-encapsulating a COX-2 inhibitor and a herbal compound for treatment of lung cancer. Eur J Pharm Biopharm Elsevier. 2016;103:1–12.
Hinds WC. Aerosol technology : properties, behavior, and measurement of airborne particles. Wiley; 1999.
Abdelrahim ME, Chrystyn H. Aerodynamic characteristics of nebulized terbutaline sulphate using the next generation impactor (NGI) and CEN method. J Aerosol Med Pulm Drug Deliv. Mary Ann Liebert, Inc. 2 Madison Avenue Larchmont, NY 10538 USA ; 2009;22:19–28.
Yerlikaya F, Ozgen A, Vural I, Guven O, Karaagaoglu E, Khan MA, et al. Development and evaluation of paclitaxel nanoparticles using a quality-by-design approach. J Pharm Sci Elsevier. 2013;102:3748–61.
Hu X, Yang FF, Liu CY, Ehrhardt C, Liao YH. In vitro uptake and transport studies of PEG-PLGA polymeric micelles in respiratory epithelial cells. Eur J Pharm Biopharm Elsevier. 2017;114:29–37.
El-Laithy HM, Badawi A, Abdelmalak NS, El-Sayyad N. Cubosomes as oral drug delivery systems: a promising approach for enhancing the release of clopidogrel bisulphate in the intestine. Chem Pharm Bull. 2018;66:1165–73.
Koay MA, Gao X, Washington MK, Parman KS, Sadikot RT, Blackwell TS, et al. Macrophages are necessary for maximal nuclear factor-κB activation in response to endotoxin. Am J Respir Cell Mol Biol. 2002;26:572–8.
Sonoda K, Ohtake K, Tagiri M, Hirata M, Tamada H, Uchida H, et al. Dietary nitrite attenuates elastase-induced pulmonary emphysema in a mouse model. Biol Pharm Bull. 2018;41:1818–23.
Bancroft JD, Gamble M. Theory and practice of histological techniques. Churchill Livingstone. 2008;725.
Palmer JA, Abberton KM, Mitchell GM, Morrison WA. Macrophage phenotype in response to implanted synthetic scaffolds: an immunohistochemical study in the rat. Cells Tissues Organs. 2014;199:169–83.
Menon P, Fisher EA. Immunostaining of macrophages, endothelial cells, and smooth muscle cells in the atherosclerotic Mouse Aorta. Methods Mol Biol. 2015. p. 131–48.
Ali M. Pulmonary drug delivery. Handb Non-Invasive Drug Deliv Syst. Elsevier; 2010. p. 209–246.
Lewis D, Copley M. Inhaled product characterization calculating particle-size distribution metrics.
Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov Nature Publishing Group. 2007;6:67–74.
Ong HX, Traini D, Young PM. Pharmaceutical applications of the Calu-3 lung epithelia cell line. Expert Opin Drug Deliv Taylor & Francis. 2013;10:1287–302.
Soliman ME, Elmowafy E, Casettari L, Alexander C. Star-shaped poly(oligoethylene glycol) copolymer-based gels: thermo-responsive behaviour and bioapplicability for risedronate intranasal delivery. Int J Pharm. 2018;543:224–33.
Grenha A, Grainger CI, Dailey LA, Seijo B, Martin GP, Remuñán-López C, et al. Chitosan nanoparticles are compatible with respiratory epithelial cells in vitro. Eur J Pharm Sci. 2007;31:73–84.
Sivadas N, O’Rourke D, Tobin A, Buckley V, Ramtoola Z, Kelly JG, et al. A comparative study of a range of polymeric microspheres as potential carriers for the inhalation of proteins. Int J Pharm Elsevier. 2008;358:159–67.
Mohammed M, Syeda J, Wasan K, Wasan E. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics. 2017;9:53.
Nasr M, Awad GAS, Mansour S, Shamy AA, Mortada ND. Hydrophilic versus hydrophobic porogens for engineering of poly(lactide-co-glycolide) microparticles containing risedronate sodium. Pharm Dev Technol. 2013;18:1078–88.
Chono S, Tanino T, Seki T, Morimoto K. Influence of particle size on drug delivery to rat alveolar macrophages following pulmonary administration of ciprofloxacin incorporated into liposomes. J Drug Target. 2006;14:557–66.
Snider GL, Lucey EC, Stone PJ. Animal models of emphysema 1– 3. Am Rev Respir Dis 2015;133:149–169.
Zhang X, Zheng H, Zhang H, Ma W, Wang F, Liu C, et al. Increased interleukin (IL)-8 and decreased IL-17 production in chronic obstructive pulmonary disease (COPD) provoked by cigarette smoke. Cytokine Elsevier Ltd. 2011;56:717–25.
Vlachaki EM, Koutsopoulos AV, Tzanakis N, Neofytou E, Siganaki M, Drositis I, et al. Altered surfactant protein-A expression in type II pneumocytes in COPD. Chest American College of Chest Physicians. 2010;137:37–45.
Duan M, Steinfort DP, Smallwood D, Hew M, Chen W, Ernst M, et al. CD11b immunophenotyping identifies inflammatory profiles in the mouse and human lungs. Mucosal Immunol Nature Publishing Group. 2016;9:550–63.
Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GRS, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol. 2013;49:503–10.
Thompson K, Rogers MJ, Coxon FP, Crockett JC. Cytosolic entry of bisphosphonate drugs requires acidification of vesicles after fluid-phase endocytosis. Mol Pharmacol Mol Pharmacol. 2006;69:1624–32.
Rogers M. New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des. 2003;9:2643–58.
Van Oort M. In vitro testing of dry powder inhalers. Aerosol Sci Technol. 1995;22(4):364–73.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to this work and participated in the following:
- The conception or design of the work; the acquisition, analysis, or interpretation of data
- Drafting the work and revising it critically for important intellectual content
- Final approval of the version to be published
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elkady, O.A., Saleh, L.M., Tadros, M.I. et al. Nebulization of Risedronate Sodium Microspheres for Potential Attenuation of Pulmonary Emphysema: a Promising New Insight of Alveolar Macrophage Apoptosis. AAPS PharmSciTech 22, 202 (2021). https://doi.org/10.1208/s12249-021-02078-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02078-8