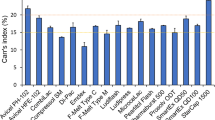
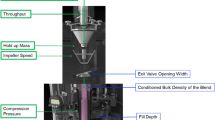
Abstract
In direct compression of tablets, it is crucial to maintain content uniformity within acceptable margins, especially in formulations with low drug loading. To assure it, complex and multistep mixing processes are utilized in the industry. In this study, we suggest the use of a simple segregation test to evaluate mixing process performance and mixture segregation to produce tablets having satisfying content uniformity while keeping the process as simple and low cost as possible. Eventually, the formulation propensity to segregation can be evaluated using process analytical technology (PAT) to adjust the mixing process parameters to changing source drug properties. In this study, that approach was examined on a model drug with a broad batch-to-batch variability in particle size and shape. Excipients were chosen so that the resulting blend composition mimicked some marketed formulations. For each drug batch, two formulation blends were prepared through different preparation processes (one simple and one complex) and subsequently subjected to segregation tests. From those, segregation coefficients were obtained to compare segregation tendencies and homogeneity robustness between the drug batches and the blend preparation methods. The inter-particulate interactions were substantially influenced by the drug particle morphology and size and resulted in different segregation behavior. Based on these findings, a simple segregation test proved to be a useful tool for determining the suitability of different batches of the model drug to be used in a certain formulation. Moreover, for a particular batch A, the test revealed a potential for mixing process simplification and therefore process intensification and cost reduction.
Graphical abstract








Similar content being viewed by others
References
Hickey AJ. Pharmaceutical process engineering: Taylor & Francis; 2001.
Mangal S, Meiser F, Morton D, Larson I. Particle engineering of excipients for direct compression: understanding the role of material properties. Curr Pharm Des. 2015;21(40):5877–89.
Nakamura S, Tanaka C, Yuasa H, Sakamoto T. Utility of microcrystalline cellulose for improving drug content uniformity in tablet manufacturing using direct powder compression. AAPS PharmSciTech. 2019;20(4):151. https://doi.org/10.1208/s12249-019-1365-4.
Li Z, Zhao L, Lin X, Shen L, Feng Y. Direct compaction: an update of materials, trouble-shooting, and application. Int J Pharm. 2017;529(1):543–56. https://doi.org/10.1016/j.ijpharm.2017.07.035.
Williams JC. The mixing of dry powders. Powder Technol. 1968;2(1):13–20. https://doi.org/10.1016/0032-5910(68)80028-2.
Jaklič M, Kočevar K, Srčič S, Dreu R. Particle size-based segregation of pharmaceutical powders in a vertical chute with a closed bottom: an experimental evaluation. Powder Technol. 2015;278:171–80. https://doi.org/10.1016/j.powtec.2015.03.021.
Liss ED, Conway SL, Zega JA, Glasser BJ. Segregation of powders during gravity flow through vertical pipes. Pharm Technol. 2004;28(2):78–97.
Alizadeh M, Hassanpour A, Pasha M, Ghadiri M, Bayly A. The effect of particle shape on predicted segregation in binary powder mixtures. Powder Technol. 2017;319:313–22. https://doi.org/10.1016/j.powtec.2017.06.059.
Swaminathan V, Kildsig DO. The effect of particle morphology on the physical stability of pharmaceutical powder mixtures: the effect of surface roughness of the carrier on the stability of ordered mixtures. Drug Dev Ind Pharm. 2000;26(4):365–73. https://doi.org/10.1081/DDC-100101242.
Brewster R, Grest GS, Levine AJ. Effects of cohesion on the surface angle and velocity profiles of granular material in a rotating drum. Phys Rev E. 2009;79(1):011305. https://doi.org/10.1103/PhysRevE.79.011305.
Crouter A, Briens L. Methods to assess mixing of pharmaceutical powders. AAPS PharmSciTech. 2019;20(2):84. https://doi.org/10.1208/s12249-018-1286-7.
Bergum JS, Prescott JK, Tejwani RW, Garcia TP, Clark J, Brown W. Current events in blend and content uniformity. Pharm Eng. 2014;34:28–39.
Mao C, Thalladi VR, Kim DK, Ma SH, Edgren D, Karaborni S. Harnessing ordered mixing to enable direct-compression process for low-dose tablet manufacturing at production scale. Powder Technol. 2013;239:290–9. https://doi.org/10.1016/j.powtec.2013.02.016.
Joshi SA, Jalalpure SS, Kempwade AA, Peram MR. Development and validation of HPLC method to determine colchicine in pharmaceutical formulations and its application for analysis of solid lipid nanoparticles. Curr Pharm Anal. 2018;14(1):76–83. https://doi.org/10.2174/1573412912666161013124417.
Paris I, Janoly-Dumenil A, Paci A, Mercier L, Bourget P, Brion F, et al. Near infrared spectroscopy and process analytical technology to master the process of busulfan paediatric capsules in a university hospital. J Pharm Biomed Anal. 2006;41:1171–8. https://doi.org/10.1016/j.jpba.2006.02.049.
Sierra-Vega NO, Román-Ospino A, Scicolone J, Muzzio FJ, Romañach RJ, Méndez R. Assessment of blend uniformity in a continuous tablet manufacturing process. Int J Pharm. 2019;560:322–33. https://doi.org/10.1016/j.ijpharm.2019.01.073.
Wang H, Barona D, Oladepo S, Williams L, Hoe S, Lechuga-Ballesteros D, et al. Macro-Raman spectroscopy for bulk composition and homogeneity analysis of multi-component pharmaceutical powders. J Pharm Biomed Anal. 2017;141:180–91. https://doi.org/10.1016/j.jpba.2017.04.003.
Sen K, Velez N, Anderson C, Drennen Iii JK, Zidan AS, Chaudhuri B. Multicomponent granular mixing in a Bohle bin blender-experiments and simulation. Int J Pharm. 2020;578:119131. https://doi.org/10.1016/j.ijpharm.2020.119131.
Baxter T, Prescott J. Process development, optimization, and scale-up: powder handling and segregation concerns. Developing Solid Oral Dosage Forms. 2009:637–65.
Zámostný P, Patera J, Hušková P. Device for the particulate solid mixture segregation propensity evaluation. Utility model CZ 22553. 2011.
Tang P, Puri VM. Methods for minimizing segregation: a review. Part Sci Technol. 2004;22(4):321–37. https://doi.org/10.1080/02726350490501420.
Shah KR, Farag Badawy SI, Szemraj MM, Gray DB, Hussain MA. Assessment of segregation potential of powder blends. Pharm Dev Technol. 2007;12(5):457–62. https://doi.org/10.1080/10837450701556834.
Saharan VA, Kukkar V, Kataria M, Kharb V, Choudhury P. Ordered mixing: mechanism, process and applications in pharmaceutical formulations. Asian J Pharm Sci. 2008;3(6):240–59.
Acknowledgements
The authors would like to thank Adam Vít and David Novák at the University of Chemistry and Technology Prague for their assistance with some of the experiments.
Funding
This work was supported by the grant of specific university research (MSMT No 21-SVV/2021)—grant No. A2_FCHT_2021_043. Technical and scientific support was also provided from The Parc.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Simona Römerová, under the supervision of Petr Zámostný. The first draft of the manuscript was written by Simona Römerová. Petr Zámostný and Ondřej Dammer reviewed, edited, and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Fig. 1
Main used excipients SEM images. Microcrystalline cellulose, upper left; pregelatinized corn starch, upper right; crospovidone, bottom. 200x mag (PNG 30419 kb)
Supplementary Fig. 2
Model mixtures SEM images. Batch A, upper left; batch B, upper right; batch C, bottom left; batch D, bottom right (drug particles are darker gray needles or columns). 300x mag (PNG 52921 kb)
Rights and permissions
About this article
Cite this article
Römerová, S., Dammer, O. & Zámostný, P. Streamlining of the Powder Mixing Process based on a Segregation Test. AAPS PharmSciTech 22, 190 (2021). https://doi.org/10.1208/s12249-021-02073-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02073-z