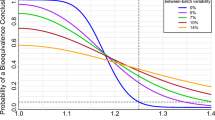
Abstract
Pharmacokinetic differences between manufacturing batches, well established for inhaled drug products, preclude control of patient risk in the customary two-way (single batch) pharmacokinetic bioequivalence crossover design if batches are randomly chosen. European regulators have recommended selecting a “typical” in vitro batch to represent each product in pharmacokinetic bioequivalence testing. We explored the feasibility of this approach to control patient risk (the “false equivalence”, or Type I, error rate). The probability of achieving a Test/Reference 90% confidence interval within (0.80, 1.25) for a true (non-equivalent) value of 1.25 was simulated for a two-way crossover design using the median in vitro batch across a range of number of in vitro batches, in vitro/in vivo correlation (IVIVC) quality (correlation coefficient, r, of zero to one), and within-subject between-batch pharmacokinetic variability. Even under extremely optimistic conditions, e.g., r=0.95 and >100 batches per product screened in vitro, patient risk for typical between-batch variability levels remained at least threefold higher than the 5% regulatory expectation for the significance level (the false equivalence error rate) of the pharmacokinetic bioequivalence test. This elevated error rate in bioequivalence decision-making occurs because of incomplete confidence that the true product average has been identified, and, importantly, omission of this uncertainty from the bioequivalence confidence interval.

Similar content being viewed by others
Change history
01 September 2021
Reference #8 has been updated.
References
Evans C, Cipolla D, Chesworth T, Agurell E, Ahrens R, Conner D, et al. Equivalence considerations for orally inhaled products for local action – ISAM/IPAC-RS European workshop report. J Aerosol Med Pulm Drug Deliv. 2012;25(3):117–39.
Hochhaus G, Horhota S, Hendeles L, Suarez S, Rebello J. Pharmacokinetics of orally inhaled drug products. AAPS J. 2015;17(3):769–75.
Questions & Answers: positions on specific questions addressed to the Pharmacokinetics Working Party (PKWP). European Medicines Agency, Committee for Human Medicinal Products, January 22, 2015. <http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002963.pdf>
Burmeister Getz E, Carroll KJ, Jones B, Benet LZ. Batch-to-batch pharmacokinetic variability confounds current bioequivalence regulations: a dry powder inhaler randomized clinical trial. Clin Pharmacol Ther. 2016;100(3):223–31.
Burmeister Getz E, Carroll KJ, Mielke J, Benet LZ, Jones B. Between-batch pharmacokinetic variability inflates Type I error rate in conventional bioequivalence trials: a randomized Advair Diskus clinical trial. Clin Pharmacol Ther. 2017;101(3):331–40.
Lähelmä S, Sairanen U, Haikarainen J, Korhonen J, Vahteristo M, Fuhr R, et al. Equivalent lung dose and systemic exposure of budesonide/formoterol combination via Easyhaler and Turbuhaler. J Aerosol Med Pulm Drug Deliv. 2015;28(6):462–73.
Burmeister Getz E, Carroll KJ, Mielke J, Jones B, Benet LZ. Pharmacokinetic behavior of fluticasone propionate and salmeterol from Advair Diskus: the consequences of batch variability. Respir Drug Deliv. 2017;1:25–34.
Burmeister Getz E, Carroll KJ, Christopher D, Morgan B, Haughie S, Cavecchi A, et al. Performance of multiple-batch approaches to pharmacokinetic bioequivalence testing for orally-inhaled drug products with batch-to-batch variability. https://doi.org/10.1208/s12249-021-02063-1
Sandell D, Olsson B, Borgström L. PK bioequivalence testing when between-batch variability is high: a multiple-batch proposal. Inhalation. 2017;11(6):13–9.
Bäckman P, Tehler U, Olsson B. Predicting exposure after oral inhalation of the selective glucocorticoid receptor modulator, AZD5423, based on dose deposition pattern, and mechanistic modeling of pulmonary deposition. J Aerosol Med Pulm Drug Deliv. 2017;30(2):108–17.
Davit BM, Nwakama PE, Buehler GJ, Conner D, Haidar SH, Patel DT, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43(10):1583–97.
Forbes B, Bäckman P, Christopher D, Dolovich M, Li BV, Morgan B. In vitro testing for orally inhaled products: developments in science-based regulatory approaches. AAPS J. 2015;17(4):837–52.
Hochhaus G, Davis-Cutting C, Oliver M, Lee SL, Lyapustina S. Current scientific and regulatory approaches for development of orally inhaled and nasal drug products: overview of the IPAC-RS/University of Florida inhalation conference. AAPS J. 2015;17(5):1305–11.
Lee SL, Saluja B, García-Arieta A, Santos GML, Li Y, Lu S, et al. Regulatory considerations for approval of generic inhalation drug products in the US, EU, Brazil, China, and India. AAPS J. 2015;17(5):1285–304.
Weber B, Hochhaus G. A pharmacokinetic simulation tool for inhaled corticosteroids. AAPS J. 2013;15(1):159–71.
Bhagwat S, Schilling U, Chen MJ, Wei X, Delvadia R, Absar M, et al. Predicting pulmonary pharmacokinetics from in vitro properties of dry powder inhalers. Pharm Res. 2017;34:2541–56.
Martin AR, Finlay WH. Model calculations of regional deposition and disposition for single doses of inhaled liposomal and dry powder ciprofloxacin. J Aerosol Med Pulm Drug Deliv 2017;30(0):1-12.
Byron PR, Weers JG, Clark AR, Sandell D, Mitchell JP. Achieving deposition equivalence: the state of the art. Respir Drug Deliv. 2017;1:101–18.
Guidance for industry: extended release oral dosage forms: development, evaluation, and application of in vitro/in vivo correlations. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), September 1997.
Guidance for industry: waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), December 2017.
Acknowledgements
E.B.G. is an employee of Oriel Therapeutics, an indirect wholly owned subsidiary of Novartis Pharma AG. K.J.C. and L.Z.B. are paid consultants to Oriel Therapeutics. B.J. and J.M. are employees of Novartis Pharma AG. J.M. was supported by the Swiss State Secretariat for Education, Research and Innovation, contract number 999754557. Opinions expressed and arguments employed herein do not necessarily reflect the official views of the Swiss Government. J.M.’s work is part of the IDEAS European training network (http://www.ideas-itn.eu/) from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No. 633567. The work was paid for by Oriel Therapeutics.
Funding
All authors were employed by their indicated affiliated company at the time of this work. K.J.C. and L.Z.B are paid consultants to Oriel Therapeutics.
Author information
Authors and Affiliations
Contributions
All authors contributed to development of the work scope, interpretation of results, and preparation of the article. K.J.C. developed the statistical framework and performed the simulations. J.M. and B.J. confirmed the statistical methodology.
Corresponding author
Ethics declarations
Conflict of Interest
The authors, excluding K.J.C and L.Z.B., are employed by companies that perform, interpret, and report pharmacokinetic bioequivalence testing of inhaled drug products.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Burmeister Getz, E., Carroll, K.J., Mielke, J. et al. Batch Selection via In Vitro/In Vivo Correlation in Pharmacokinetic Bioequivalence Testing. AAPS PharmSciTech 22, 224 (2021). https://doi.org/10.1208/s12249-021-02064-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02064-0