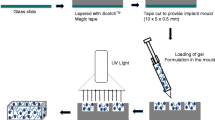
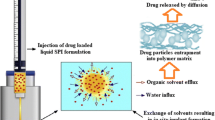
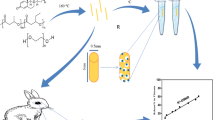
Abstract
Posterior eye diseases are a common cause of vision problems in developing countries, which have encouraged the development of new treatment models for these degenerative diseases. Intraocular implants are one of the drug delivery systems to the posterior region of the eye. Using these implants, the blood-eye barrier can be bypassed; the complications caused by repeated in vitro administrations can be eliminated, and smaller amounts of the drug would be used during the treatment process. Meanwhile, biodegradable implants have received more attention due to their biodegradable structure and the lack of need for re-surgery to remove the rest of the system from the eye. The aim of this study is to employ biodegradable implants composed of polyethylene glycol (PEG) and 3-hydroxybutyrate-co-3-hydroxyvalerat (PHBV) to deliver betamethasone to the back of the eye in the treatment of retinopathy. PHBV polymer has been selected as the main polymer with a certain ratio of drug to polymer for fabrication of enamel and different amounts of PEG with three molecular weights used as pore generators to control drug release over a period of time. Based on the analysis of the results of differential scanning calorimetry (DSC) and FTIR spectroscopy, none of the polymers were degraded in the temperature range of the manufacturing process, and among betamethasone derivatives, the best option for implant preparation is the use of its basic form. Drug release studies over a period of three months showed that implants containing PHBV HV2% and PEG 6000 had a more appropriate release profile.







Similar content being viewed by others
References
Sabanayagam C, Banu R, Chee ML, Lee R, Wang YX, Tan G, et al. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diab Endocrinol. 2019;7(2):140–9.
Heckenlively JR, Ferreyra HA, editors. Autoimmune retinopathy: a review and summary. Semin Immunopathol. 2008;Springer.
Akram MU, Khalid S, Tariq A, Khan SA, Azam F. Detection and classification of retinal lesions for grading of diabetic retinopathy. Comput Biol Med. 2014;45:161–71.
Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vision. 2015;2(1):1–25.
Ding J, Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2012;12(4):346–54.
Sabanayagam C, Yip W, Ting DS, Tan G, Wong TY. Ten emerging trends in the epidemiology of diabetic retinopathy. Ophthalmic Epidemiol. 2016;23(4):209–22.
Ludwig CA, Chen TA, Hernandez-Boussard T, Moshfeghi AA, Moshfeghi DM. The epidemiology of retinopathy of prematurity in the United States. Ophthalmic Surg Lasers Imaging Retin. 2017;48(7):553–62.
Gupta N, Mansoor S, Sharma A, Sapkal A, Sheth J, Falatoonzadeh P, et al. Diabetic retinopathy and VEGF. Open Ophthalmol J. 2013;7:4–10.
Hofman P, Blaauwgeers H, Vrensen G, Schlingemann R. Role of VEGF-A in endothelial phenotypic shift in human diabetic retinopathy and VEGF-A-induced retinopathy in monkeys. Ophthalmic Res. 2001;33(3):156–62.
Ray D, Mishra M, Ralph S, Read I, Davies R, Brenchley P. Association of the VEGF gene with proliferative diabetic retinopathy but not proteinuria in diabetes. Diabetes. 2004;53(3):861–4.
Schwartz GS, Flynn WH. Fluocinolone acetonide implantable device for diabetic retinopathy. Curr Pharm Biotechnol. 2011;12(3):347–51.
Wykoff CC. Impact of intravitreal pharmacotherapies including antivascular endothelial growth factor and corticosteroid agents on diabetic retinopathy. Curr Opin Ophthalmol. 2017;28(3):213–8.
Gupta SR, Suhler EB, Rosenbaum JT. The dilemma of central serous retinopathy, a corticosteroid-induced complication, in patients with ocular inflammatory disease. J Rheumatol. 2010;37(9):1973–4.
Jain I, Singh K. Maculopathy a corticosteroid side-effect. Indian J Ophthalmol. 1966;14(6):250.
Couvreur P. Nanoparticles in drug delivery: past, present and future. Adv Drug Deliv Rev. 2013;65(1):21–3.
Lang JC. Ocular drug delivery conventional ocular formulations. Adv Drug Deliv Rev. 1995;16(1):39–43.
Maghsoudnia N, Eftekhari RB, Sohi AN, Zamzami A, Dorkoosh FA. Application of nano-based systems for drug delivery and targeting: a review. J Nanopart Res. 2020;22(8):1–41.
Sahoo SK, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today. 2008;13(3-4):144–51.
Eftekhari RB, Maghsoudnia N, Samimi S, Zamzami A, Dorkoosh FA. Co-delivery nanosystems for cancer treatment: a review. Pharm Nanotechnol. 2019;7(2):90–112.
Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348–60.
Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: an overview. World J Pharmacol. 2013;2(2):47–64.
Cholkar K, Vadlapudi AD, Trinh HM, Mitra AK. Compositions, formulation, pharmacology, pharmacokinetics, and toxicity of topical, periocular, and intravitreal ophthalmic drugs. Ocular Pharmacol Toxicol. Springer. 2013;91-118.
Gadek T, Lee D. Topical drug delivery to the back of the eye. Drug Prod Dev Back Eye. Springer. 2011;111-24.
Sampat KM, Garg SJ. Complications of intravitreal injections. Curr Opin Ophthalmol. 2010;21(3):178–83.
Sen HN, Vitale S, Gangaputra SS, Nussenblatt RB, Liesegang TL, Levy-Clarke GA, et al. Periocular corticosteroid injections in uveitis: effects and complications. Ophthalmology. 2014;121(11):2275–86.
Wong VG, Kochinke F. Biocompatible ocular implants. Google Patents; 1995.
Haffner DS, Gille HK, Kalina CR, Cogger JJ. System and method for delivering multiple ocular implants. Google Patents; 2019.
Macoon R, Guerriero T, Chauhan A. Extended release of dexamethasone from oleogel based rods. J Colloid Interface Sci. 2019;555:331–41.
Macoon R, Robey M, Chauhan A. In Vitro release of hydrophobic drugs by oleogel rods with biocompatible gelators. Eur J Pharm Sci. 2020;152:105413.
Macoon R, Chauhan A. Ophthalmic delivery of hydrophilic drugs through drug-loaded oleogels. Eur J Pharm Sci. 2020;105634.
Kumar A, Ambiya V, Kapoor G, Arora A. Wandering Ozurdex in eyes with scleral fixated intraocular lens and its management: a report of two cases. J Curr Ophthalmol. 2019;31(3):345–8.
Xu Y, Kim CS, Saylor DM, Koo D. Polymer degradation and drug delivery in PLGA-based drug–polymer applications: a review of experiments and theories. J Biomed Mater Res B Appl Biomater. 2017;105(6):1692–716.
Yang C, Plackett D, Needham D, Burt HM. PLGA and PHBV microsphere formulations and solid-state characterization: possible implications for local delivery of fusidic acid for the treatment and prevention of orthopaedic infections. Pharm Res. 2009;26(7):1644–56.
Haghjou N, Soheilian M, Abdekhodaie MJ. Sustained release intraocular drug delivery devices for treatment of uveitis. J Ophthalmic Vision Res. 2011;6(4):317–29.
Pacella F, Ferraresi AF, Turchetti P, Lenzi T, Giustolisi R, Bottone A, et al. Intravitreal injection of Ozurdex® implant in patients with persistent diabetic macular edema, with six-month follow-up. Ophthalmol Eye Dis. 2016;8:OED. S38028.
Kiss S. Sustained-release corticosteroid delivery systems. Retin Today. 2010;44–6.
Mohtashami Z, Javar HA, Tehrani MR, Esfahani MR, Roohipour R, Aghajanpour L, et al. Fabrication, Optimization, and In Vitro and In Vivo Characterization of Intra-vitreal Implant of Budesonide Generally Made of PHBV. AAPS PharmSciTech. 2020;21(8):1–12.
Sultana N, Khan TH. In vitro degradation of PHBV scaffolds and nHA/PHBV composite scaffolds containing hydroxyapatite nanoparticles for bone tissue engineering. J Nanomater. 2012;2012:1–12.
Tebaldi ML, Maia ALC, Poletto F, de Andrade FV, Soares DCF. Poly (-3-hydroxybutyrate-co-3-hydroxyvalerate)(PHBV): current advances in synthesis methodologies, antitumor applications and biocompatibility. J Drug Deliv Sci Technol. 2019;51:115–26.
Wang S, Song C, Chen G, Guo T, Liu J, Zhang B, et al. Characteristics and biodegradation properties of poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/organophilic montmorillonite (PHBV/OMMT) nanocomposite. Polym Degrad Stab. 2005;87(1):69–76.
Jiang L, Morelius E, Zhang J, Wolcott M, Holbery J. Study of the poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/cellulose nanowhisker composites prepared by solution casting and melt processing. J Compos Mater. 2008;42(24):2629–45.
Citirik M, Dilsiz N, Batman C, Zilelioglu O. Comparative toxicity of 4 commonly used intravitreal corticosteroids on rat retina. Can J Ophthalmol. 2009;44(3):e3–8.
Fruijtier-Pölloth C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology. 2005;214(1-2):1–38.
Avella M, Martuscelli E, Raimo M. Review Properties of blends and composites based on poly (3-hydroxy) butyrate (PHB) and poly (3-hydroxybutyrate-hydroxyvalerate)(PHBV) copolymers. J Mater Sci. 2000;35(3):523–45.
Wang S, Ma P, Wang R, Wang S, Zhang Y, Zhang Y. Mechanical, thermal and degradation properties of poly (d, l-lactide)/poly (hydroxybutyrate-co-hydroxyvalerate)/poly (ethylene glycol) blend. Polym Degrad Stab. 2008;93(7):1364–9.
Wu IY, Bala S, Škalko-Basnet N, Di Cagno MP. Interpreting non-linear drug diffusion data: utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur J Pharm Sci. 2019;138:105026.
Sinko PJ, Singh Y. Martin's physical pharmacy and pharmaceutical sciences: physical chemical and biopharmaceutical principles in the pharmaceutical sciences. Walter Kluer; 2011.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Guest Editors: Qingguo Xu and Iok-Hou Pang
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Rastegar Ramsheh, Z.S., Mohtashami, Z., Kargar, N. et al. Design and In Vitro Evaluation of a Slow-Release Intraocular Implant of Betamethasone. AAPS PharmSciTech 22, 174 (2021). https://doi.org/10.1208/s12249-021-02048-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02048-0