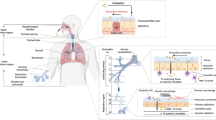
Abstract
Respiratory diseases are among the leading causes of morbidity and mortality worldwide. Innovations in biochemical engineering and understanding of the pathophysiology of respiratory diseases resulted in the development of many therapeutic proteins and peptide drugs with high specificity and potency. Currently, protein and peptide drugs are mostly administered by injections due to their large molecular size, poor oral absorption, and labile physicochemical properties. However, parenteral administration has several limitations such as frequent dosing due to the short half-life of protein and peptide in blood, pain on administration, sterility requirement, and poor patient compliance. Among various noninvasive routes of administrations, the pulmonary route has received a great deal of attention and is a better alternative to deliver protein and peptide drugs for treating respiratory diseases and systemic diseases. Among the various aerosol dosage forms, dry powder inhaler (DPI) systems appear to be promising for inhalation delivery of proteins and peptides due to their improved stability in solid state. This review focuses on the development of DPI formulations of protein and peptide drugs using advanced spray drying. An overview of the challenges in maintaining protein stability during the drying process and stabilizing excipients used in spray drying of proteins and peptide drugs is discussed. Finally, a summary of spray-dried DPI formulations of protein and peptide drugs, their characterization, various DPI devices used to deliver protein and peptide drugs, and current clinical status are discussed.




Similar content being viewed by others
References
Campbell M. Biochemistry (3rd edition). Orlando, Florida, USA: Harcourt Brace & Company; 1999.
Shoyele SA, Slowey A. Prospects of formulating proteins/peptides as aerosols for pulmonary drug delivery. Int J Pharm. 2006;314(1):1–8. https://doi.org/10.1016/j.ijpharm.2006.02.014.
Wan F, Møller EH, Yang M, Jørgensen L. Formulation technologies to overcome unfavorable properties of peptides and proteins for pulmonary delivery. Drug Discov Today Technol. 2012;9(2):e141-e6. https://doi.org/10.1016/j.ddtec.2011.12.003.
Crompton G. A brief history of inhaled asthma therapy over the last fifty years. Prim Care Resp J. 2006;15(6):326–31. https://doi.org/10.1016/j.pcrj.2006.09.002.
Stein SW, Thiel CG. The history of therapeutic aerosols: a chronological review. J Aerosol Med Pulm Drug Deliv. 2017;30(1):20–41. https://doi.org/10.1089/jamp.2016.1297.
Eedara BB, Tucker IG, Zujovic ZD, Rades T, Price JR, Das SC. Crystalline adduct of moxifloxacin with trans-cinnamic acid to reduce the aqueous solubility and dissolution rate for improved residence time in the lungs. Eur J Pharm Sci. 2019;136:104961. https://doi.org/10.1016/j.ejps.2019.104961.
Hickey AJ. Back to the future: inhaled drug products. J Pharm Sci. 2013;102(4):1165–72.
Rau JL. The inhalation of drugs: advantages and problems. Respir Care. 2005;50(3):367–82.
Eedara BB, Tucker IG, Das SC. In vitro dissolution testing of respirable size anti-tubercular drug particles using a small volume dissolution apparatus. Int J Pharm. 2019;559:235–44. https://doi.org/10.1016/j.ijpharm.2019.01.035.
Garcia-Contreras L, Smyth HDC. Liquid-spray or dry-powder systems for inhaled delivery of peptide and proteins? Am J Drug Deliv. 2005;3(1):29–45. https://doi.org/10.2165/00137696-200503010-00004.
Weers JG, Tarara TE, Clark AR. Design of fine particles for pulmonary drug delivery. Expert Opin Drug Deliv. 2007;4(3):297–313. https://doi.org/10.1517/17425247.4.3.297.
Moon C, Smyth HD, Watts AB, Williams RO. Delivery technologies for orally inhaled products: an update. AAPS PharmSciTech. 2019;20(3):117.
Pilcer G, Amighi K. Formulation strategy and use of excipients in pulmonary drug delivery. Int J Pharm. 2010;392(1-2):1–19.
Shetty N, Cipolla D, Park H, Zhou QT. Physical stability of dry powder inhaler formulations. Expert Opin Drug Deliv. 2020;17(1):77–96.
Chow AH, Tong HH, Chattopadhyay P, Shekunov BY. Particle engineering for pulmonary drug delivery. Pharm Res. 2007;24(3):411–37.
Ameri M, Maa Y-F. Spray drying of biopharmaceuticals: stability and process considerations. Dry Technol. 2006;24(6):763–8.
Lee G. Spray-drying of proteins. In: Carpenter JF, Manning MC, Editors. Rational design of stable protein formulations: theory and practice. Boston, MA: Springer US; 2002. p. 135-58.
Haggag YA, Faheem AM. Evaluation of nano spray drying as a method for drying and formulation of therapeutic peptides and proteins. Front Pharmacol. 2015;6.
Sarabandi K, Gharehbeglou P, Jafari SM. Spray-drying encapsulation of protein hydrolysates and bioactive peptides: opportunities and challenges. Dry Technol. 2020;38(5-6):577–95.
Irngartinger M, Camuglia V, Damm M, Goede J, Frijlink H. Pulmonary delivery of therapeutic peptides via dry powder inhalation: effects of micronisation and manufacturing. Eur J Pharm Biopharm. 2004;58(1):7–14.
Greb E. Is spray drying a viable alternative to lyophilization. Equipment and Processing Report. 2009.
Singh A, Van den Mooter G. Spray drying formulation of amorphous solid dispersions. Adv Drug Deliv Rev. 2016;100:27–50. https://doi.org/10.1016/j.addr.2015.12.010
Sollohub K, Cal K. Spray drying technique: II. Current applications in pharmaceutical technology. J Pharm Sci. 2010;99(2):587–97. https://doi.org/10.1002/jps.21963.
Schuck P. Spray drying of dairy products: state of the art. Le Lait. 2002;82(4):375–82.
Shoyele SA, Cawthorne S. Particle engineering techniques for inhaled biopharmaceuticals. Adv Drug Deliv Rev. 2006;58(9):1009–29. https://doi.org/10.1016/j.addr.2006.07.010.
Rosenberg M, Kopelman I, Talmon Y. Factors affecting retention in spray-drying microencapsulation of volatile materials. J Agric Food Chem. 1990;38(5):1288–94.
Shukla D, Schneider CP, Trout BL. Molecular level insight into intra-solvent interaction effects on protein stability and aggregation. Adv Drug Deliv Rev. 2011;63(13):1074–85. https://doi.org/10.1016/j.addr.2011.06.014.
Maltesen MJ, van de Weert M. Drying methods for protein pharmaceuticals. Drug Discov Today Technol. 2008;5(2):e81–e8. https://doi.org/10.1016/j.ddtec.2008.11.001.
Maa Y-F, Costantino HR, Nguyen P-A, Hsu CC. The effect of operating and formulation variables on the morphology of spray-dried protein particles. Pharm Dev Technol. 1997;2(3):213–23. https://doi.org/10.3109/10837459709031441.
Prinn KB, Costantino HR, Tracy M. Statistical modeling of protein spray drying at the lab scale. AAPS PharmSciTech. 2002;3(1):E4. https://doi.org/10.1208/pt030104.
Labrude P, Rasolomanana M, Vigneron C, Thirion C, Chaillot B. Protective effect of sucrose on spray drying of oxyhemoglobin. J Pharm Sci. 1989;78(3):223–9. https://doi.org/10.1002/jps.2600780311.
Ståhl K, Claesson M, Lilliehorn P, Lindén H, Bäckström K. The effect of process variables on the degradation and physical properties of spray dried insulin intended for inhalation. Int J Pharm. 2002;233(1):227-237. doi: https://doi.org/10.1016/S0378-5173(01)00945-0x.
Adler M, Lee G. Stability and surface activity of lactate dehydrogenase in spray-dried trehalose. J Pharm Sci. 1999;88(2):199–208. https://doi.org/10.1021/js980321x.
Mumenthaler M, Hsu CC, Pearlman R. Feasibility study on spray-drying protein pharmaceuticals: recombinant human growth hormone and tissue-type plasminogen activator. Pharm Res. 1994;11(1):12–20. https://doi.org/10.1023/A:1018929224005.
Banga A. Lyophilization, pharmaceutical processing, and handling of therapeutic peptides and proteins. In Therapeutic Peptides and Proteins. 3rd ed. CRC Press, 2006:147-75.
Maa YF, Prestrelski SJ. Biopharmaceutical powders: particle formation and formulation considerations. Curr Pharm Biotechnol. 2000;1(3):283–302. https://doi.org/10.2174/1389201003378898.
Grasmeijer N. Improving protein stabilization by spray drying [PhD thesis]. University of Groningen. 2016.
Depreter F, Pilcer G, Amighi K. Inhaled proteins: challenges and perspectives. Int J Pharm. 2013;447(1):251–80. https://doi.org/10.1016/j.ijpharm.2013.02.031.
Costantino HR, Andya JD, Nguyen P-A, Dasovich N, Sweeney TD, Shire SJ, et al. Effect of mannitol crystallization on the stability and aerosol performance of a spray-dried pharmaceutical protein, recombinant humanized anti-IgE monoclonal antibody. J Pharm Sci. 1998;87(11):1406–11. https://doi.org/10.1021/js9800679.
Faghihi H, Vatanara A, Najafabadi AR, Ramezani V, Gilani K. The use of amino acids to prepare physically and conformationally stable spray-dried IgG with enhanced aerosol performance. Int J Pharm. 2014;466(1-2):163–71. https://doi.org/10.1016/j.ijpharm.2014.03.020.
Johnson KA. Preparation of peptide and protein powders for inhalation. Adv Drug Deliv Rev. 1997;26(1):3–15. https://doi.org/10.1016/S0169-409X(97)00506-1.
Ajmera A, Scherließ R. Stabilisation of proteins via mixtures of amino acids during spray drying. Int J Pharm. 2014;463(1):98–107. https://doi.org/10.1016/j.ijpharm.2014.01.002.
Pikal-Cleland KA, Cleland JL, Anchordoquy TJ, Carpenter JF. Effect of glycine on pH changes and protein stability during freeze–thawing in phosphate buffer systems. J Pharm Sci. 2002;91(9):1969–79.
Platz RM, Brewer TK, Boardman TD. Dispersible macromolecule compositions and methods for their preparation and use: Google Patents; 2003.
Hasija M, Li L, Rahman N, Ausar SF. Forced degradation studies: an essential tool for the formulation development of vaccines. Vaccine: Development and Therapy. 2013;3:11–33.
Chang L, Pikal MJ. Mechanisms of protein stabilization in the solid state. J Pharm Sci. 2009;98(9):2886–908. https://doi.org/10.1002/jps.21825.
Eedara BB, Rangnekar B, Doyle C, Cavallaro A, Das SC. The influence of surface active l-leucine and 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC) in the improvement of aerosolization of pyrazinamide and moxifloxacin co-spray dried powders. Int J Pharm. 2018;542(1-2):72–81. https://doi.org/10.1016/j.ijpharm.2018.03.005.
Eedara BB, Rangnekar B, Sinha S, Doyle C, Cavallaro A, Das SC. Development and characterization of high payload combination dry powders of anti-tubercular drugs for treating pulmonary tuberculosis. Eur J Pharm Sci. 2018;118:216–26. https://doi.org/10.1016/j.ejps.2018.04.003.
Rangnekar B, Momin MAM, Eedara BB, Sinha S, Das SC. Bedaquiline containing triple combination powder for inhalation to treat drug-resistant tuberculosis. Int J Pharm. 2019;570:118689. https://doi.org/10.1016/j.ijpharm.2019.118689.
Eedara BB, Tucker IG, Das SC. Phospholipid-based pyrazinamide spray-dried inhalable powders for treating tuberculosis. Int J Pharm. 2016;506(1):174–83. https://doi.org/10.1016/j.ijpharm.2016.04.038.
Quarta E, Chierici V, Flammini L, Tognolini M, Barocelli E, Cantoni AM, et al. Excipient-free pulmonary insulin dry powder: pharmacokinetic and pharmacodynamics profiles in rats. J Control Release. 2020;323:412–20. https://doi.org/10.1016/j.jconrel.2020.04.015.
Xia Y, Su Y, Wang Q, Yang C, Tang B, Zhang Y, et al. Preparation, characterization, and pharmacodynamics of insulin-loaded fumaryl diketopiperazine microparticle dry powder inhalation. Drug Deliv. 2019;26(1):650–60.
Chan H-K, Clark AR, Feeley JC, Kuo M-C, Russ Lehrman S, Pikal-Cleland K, et al. Physical stability of salmon calcitonin spray-dried powders for inhalation. J Pharm Sci. 2004;93(3):792–804. https://doi.org/10.1002/jps.10594.
Yang M, Velaga S, Yamamoto H, Takeuchi H, Kawashima Y, Hovgaard L, et al. Characterisation of salmon calcitonin in spray-dried powder for inhalation: effect of chitosan. Int J Pharm. 2007;331(2):176–81. https://doi.org/10.1016/j.ijpharm.2006.10.030.
Tewes F, Tajber L, Corrigan O, Ehrhardt C, Healy A-MJEJPS. Development and characterisation of soluble polymeric particles for pulmonary peptide delivery. Eur J Pharm Sci. 2010;41(2):337–52. https://doi.org/10.1016/j.ejps.2010.07.001.
Tewes F, Gobbo OL, Amaro MI, Tajber L, Corrigan OI, Ehrhardt C, et al. Evaluation of HPβCD-PEG microparticles for salmon calcitonin administration via pulmonary delivery. Mol Pharm. 2011;8(5):1887–98. https://doi.org/10.1021/mp200231c.
Wang L, Zhang Y, Tang X. Characterization of a new inhalable thymopentin formulation. Int J Pharm. 2009;375(1-2):1–7. https://doi.org/10.1016/j.ijpharm.2009.03.020.
Li HY, Song X, Seville PC. The use of sodium carboxymethylcellulose in the preparation of spray-dried proteins for pulmonary drug delivery. Eur J Pharm Sci. 2010;40(1):56–61. https://doi.org/10.1016/j.ejps.2010.02.007.
Wu X, Zhang W, Hayes D Jr, Mansour HM. Physicochemical characterization and aerosol dispersion performance of organic solution advanced spray-dried cyclosporine A multifunctional particles for dry powder inhalation aerosol delivery. Int J Nanomedicine. 2013;8:1269–83. https://doi.org/10.2147/ijn.s40904.
Park CW, Li X, Vogt FG, Hayes D Jr, Zwischenberger JB, Park ES, et al. Advanced spray-dried design, physicochemical characterization, and aerosol dispersion performance of vancomycin and clarithromycin multifunctional controlled release particles for targeted respiratory delivery as dry powder inhalation aerosols. Int J Pharm. 2013;455(1-2):374–92. https://doi.org/10.1016/j.ijpharm.2013.06.047.
Liang W, Kwok PCL, Chow MYT, Tang P, Mason AJ, Chan H-K, et al. Formulation of pH responsive peptides as inhalable dry powders for pulmonary delivery of nucleic acids. Eur J Pharm Biopharm. 2014;86(1):64–73. https://doi.org/10.1016/j.ejpb.2013.05.006.
Liang W, Chow MY, Lau PN, Zhou QT, Kwok PC, Leung GP, et al. Inhalable dry powder formulations of siRNA and pH-responsive peptides with antiviral activity against H1N1 influenza virus. Mol Pharm. 2015;12(3):910–21. https://doi.org/10.1021/mp500745v.
Kwok PCL, Grabarek A, Chow MYT, Lan Y, Li JCW, Casettari L, et al. Inhalable spray-dried formulation of D-LAK antimicrobial peptides targeting tuberculosis. Int J Pharm. 2015;491(1):367–74. https://doi.org/10.1016/j.ijpharm.2015.07.001.
Hou A, Li L, Huang Y, Singh V, Zhu C, Pan X, et al. Fragmented particles containing octreotide acetate prepared by spray drying technique for dry powder inhalation. Drug Deliv Transl Res. 2018;8(3):693–701. https://doi.org/10.1007/s13346-018-0515-7.
Brange J, Langkjœr L. Insulin structure and stability. In: Wang YJ, Pearlman R, Editors. Stability and Characterization of Protein and Peptide Drugs: Case Histories. Boston, MA: Springer US; 1993. p. 315–50.
Sélam JL. Inhaled insulin: promises and concerns. J Diabetes Sci Technol. 2008;2(2):311–5. https://doi.org/10.1177/193229680800200225.
Patton JS. Pulmonary delivery of drugs for bone disorders. Adv Drug Deliv Rev. 2000;42(3):239–48.
Mitruka SN, Won A, McCurry KR, Zeevi A, McKaveney T, Venkataramanan R, et al. In the lung aerosol cyclosporine provides a regional concentration advantage over intramuscular cyclosporine. J Heart Lung Transplant. 2000;19(10):969–75.
Peters BM, Shirtliff ME, Jabra-Rizk MA. Antimicrobial peptides: primeval molecules or future drugs? PLoS Pathog. 2010;6(10):e1001067.
Wu X, Li X, Mansour HM. Surface analytical techniques in solid-state particle characterization: implications for predicting performance in dry powder inhalers. Invited paper. KONA Powder Part J. 2010;28:3–19.
Hickey AJ, Mansour HM, Telko MJ, Xu Z, Smyth HDC, Mulder T, et al. Physical characterization of component particles included in dry powder inhalers. I. Strategy review and static characteristics. J Pharm Sci. 2007;96(5):1282–301. https://doi.org/10.1002/jps.20916.
Mansour HM, Hickey AJ. Raman characterization and chemical imaging of biocolloidal self-assemblies, drug delivery systems, and pulmonary inhalation aerosols: a review. AAPS PharmSciTech. 2007;8(4):E99. https://doi.org/10.1208/pt0804099.
Park CW, Rhee YS, Vogt FG, Hayes DJ, Zwischenberger JB, DeLuca PP, et al. Advances in microscopy and complementary imaging techniques to assess the fate of drugs ex vivo in respiratory drug delivery: an invited paper. Adv Drug Deliv Rev. 2012;64(4):344-56.
Muralidharan P, Acosta M, Hayes DJ, Black SM, Mansour HM. Solid-state physicochemical characterization & microscopy of particles in dry powder inhalers. Inhalation. 2016;10(2):20–6.
Xu Z, Mansour HM, Hickey AJ. Particle interactions in dry powder inhaler unit processes. J Adhes Sci Technol: Special Issue on Adhesion Aspects in Pharmaceutical Sciences. 2011;25(4/5):451–82.
Hickey AJ, Mansour HM. Chapter 43: Formulation challenges of powders for the delivery of small molecular weight molecules as aerosols. In: Rathbone MJ, Hadgraft J, Roberts MS, Lane M, editors. Modified-Release Drug Delivery Technology. 2nd ed. New York: Informa Healthcare; 2008. p. 573–602.
Zijlstra GS, Hinrichs WLJ, Boer AH, Frijlink HW. The role of particle engineering in relation to formulation and de-agglomeration principle in the development of a dry powder formulation for inhalation of cetrorelix. Eur J Pharm Sci. 2004;23(2):139–49. https://doi.org/10.1016/j.ejps.2004.06.005.
Ógáin ON, Li J, Tajber L, Corrigan OI, Healy AM. Particle engineering of materials for oral inhalation by dry powder inhalers. I—Particles of sugar excipients (trehalose and raffinose) for protein delivery. Int J Pharm. 2011;405(1-2):23–35.
d’Angelo I, Casciaro B, Miro A, Quaglia F, Mangoni ML, Ungaro FJC, et al. Overcoming barriers in Pseudomonas aeruginosa lung infections: engineered nanoparticles for local delivery of a cationic antimicrobial peptide. Colloids Surf B Biointerfaces. 2015;135:717–25.doi: 10.1016/j.colsurfb.2015.08.027
Balducci AG, Cagnani S, Sonvico F, Rossi A, Barata P, Colombo G, et al. Pure insulin highly respirable powders for inhalation. Eur J Pharm Sci. 2014;51:110–7. https://doi.org/10.1016/j.ejps.2013.08.009.
Fenton C, Keating GM, Plosker GL. Novolizer®. Drugs. 2003;63(22):2437–45. https://doi.org/10.2165/00003495-200363220-00010.
Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med. 2015;10(1):13. https://doi.org/10.1186/s40248-015-0012-5.
Köhler D, Coipm J. Novolizer®: the new technology for the management of asthma therapy. Curr Opin Pulm Med. 2003;9:S11–S6.
Haidl P, Pohlmann G, Cloes RM. P238: power requirement of pressurized metered-dose and dry powder inhalers (pMDIs and DPIs). In ISAM Congress, Munich 2015.
Borgström L, Bisgaard H, O'Callaghan C, Pedersen S. Dry powder inhalers. In: Bisgaard H, O'Callaghan C, Smaldone GC, Editors. Drug Delivery to the Lung. Lung Biology in Health and Disease. Vol. 162. Marcel Dekker, New York/Basel: 2002:421–48.
Zhang X. The development of a new dry powder inhaler [Electronic Thesis and Dissertation Repository]: The University of Western Ontario; 2013.
Pasquali I, Brambilla G, Copelli D. Effect of flow rate on dose delivery of three dry powder inhalers: NEXThaler®, Turbohaler® and Diskus®. RDD Europe. 2013:1-6.
Yang MY, Verschuer J, Shi Y, Song Y, Katsifis A, Eberl S, et al. The effect of device resistance and inhalation flow rate on the lung deposition of orally inhaled mannitol dry powder. Int J Pharm. 2016;513(1-2):294-301.
White S, Bennett DB, Cheu S, Conley PW, Guzek DB, Gray S, et al. EXUBERA®: pharmaceutical development of a novel product for pulmonary delivery of insulin. Diabetes Technol Ther. 2005;7(6):896–906.
Heinemann L. The failure of exubera: are we beating a dead horse? J Diabetes Sci Technol. 2008;2(3):518–29.
Burgess G, Boyce M, Jones M, Larsson L, Main MJ, Morgan F, et al. Randomized study of the safety and pharmacodynamics of inhaled interleukin-13 monoclonal antibody fragment VR942. EBioMedicine. 2018;35:67–75.
Moss RB. Treatment of human metapneumovirus: Google Patents; 2018.
Funding
This work was supported by NIH R01HL137282 (HMM), P01HL103453 (HMM), R21AI135935 (HMM), R21AG054766 (HMM), and NIDA 5UG3DA047717 (HMM and RP).
Author information
Authors and Affiliations
Contributions
Conceptualization: Basanth Babu Eedara and Heidi M. Mansour; writing original draft preparation and editing: Basanth Babu Eedara, David Encinas, Wafaa Alabsi, Robin Polt, and Heidi M. Mansour; supervision: Heidi M. Mansour.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Guest Editors: Feng Zhang, Michael Repka and Suresh Bandari
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eedara, B.B., Alabsi, W., Encinas-Basurto, D. et al. Spray-Dried Inhalable Powder Formulations of Therapeutic Proteins and Peptides. AAPS PharmSciTech 22, 185 (2021). https://doi.org/10.1208/s12249-021-02043-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02043-5