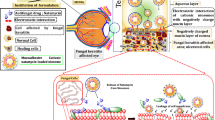
Abstract
Microbial keratitis (MK) is a vision-threatening disease and the fourth leading cause of blindness worldwide. In this work, we aim to develop moxifloxacin (MXN)-loaded chitosan-based cationic mucoadhesive polyelectrolyte nanocapsules (PENs) for the effective treatment of MK. PENs were formulated by polyelectrolyte complex coacervation method and characterized for their particle size, surface charge, morphology, mucoadhesive property, in-vitro and ex-vivo release, ocular tolerance, and antimicrobial efficacy studies. The pharmacodynamic study was conducted on rabbit eye model of induced keratitis and it is compared with marketed formulation (MF). Developed PENs showed the size range from 230.7 ± 0.64 to 249.0 ± 0.49 nm and positive surface charge, spherical shape along with appropriate physico-chemical parameters. Both in-vitro and ex-vivo examination concludes that PENs having more efficiency in sustained release of MXN compared to MF. Ocular irritation studies demonstrated that no corneal damage or ocular irritation. The in-vivo study proved that the anti-bacterial efficacy of PENs was improved when compared with MF. These results suggested that PENs are a feasible choice for MK therapy because of their ability to enhance ocular retention of loaded MXN through interaction with the corneal surface of the mucous membrane.
Graphical abstract














Similar content being viewed by others
References
O’brien TP. Management of bacterial keratitis: beyond exorcism toward consideration of organism and host factors. Eye. 2003;17:957–74. https://doi.org/10.1038/sj.eye.6700635.
Robertson DM, Rogers NA, Petroll WM, Zhu M. Second harmonic generation imaging of corneal stroma after infection by Pseudomonas aeruginosa. Sci Rep. 2017;7:46116. https://doi.org/10.1038/srep46116.
Austin A, Lietman T, Rose-Nussbaumer J. Update on the Management of Infectious Keratitis. Ophthalmology. 2017;124:1678–89. https://doi.org/10.1016/j.ophtha.2017.05.012.
Reimondez-Troitino S, Csaba N, Alonso MJ, de la Fuente M. Nanotherapies for the treatment of ocular diseases. Eur J Pharm Biopharm. 2015;95:279–93. https://doi.org/10.1016/j.ejpb.2015.02.019.
Langlois MH, Montagut M, Dubost JP, Grellet J, Saux MC. Protonation equilibrium and lipophilicity of moxifloxacin. J Pharm Biomed Anal. 2005;37:389–93. https://doi.org/10.1016/j.jpba.2004.10.022.
Balfour JA, Wiseman LR. Moxifloxacin. Drugs. 1999;57:363–74. https://doi.org/10.2165/00003495-199957030-00007.
Pestova E, Millichap JJ, Noskin GA, Peterson LR. Intracellular targets of moxifloxacin: a comparison with other fluoroquinolones. J Antimicrob Chemother. 2000;45:583–90. https://doi.org/10.1093/jac/45.5.583.
Pawar N, Bohidar HB. Anisotropic domain growth and complex coacervation in nanoclay-polyelectrolyte solutions. Adv Colloid Interf Sci. 2011;167:12–23. https://doi.org/10.1016/j.cis.2011.06.007.
Il’Ina AV, Varlamov VP. Chitosan-based polyelectrolyte complexes: a review. Appl Biochem Microbiol. 2005;41:5–11. https://doi.org/10.1007/s10438-005-0002-z.
Luo Y, Wang Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int J Biol Macromol. 2014;64:353–67. https://doi.org/10.1016/j.ijbiomac.2013.12.017.
Unagolla JM, Adikary SU. Adsorption characteristics of cadmium and lead heavy metals into locally synthesized Chitosan Biopolymer. Trop Agric Res. 2015;26:395–401. https://doi.org/10.4038/tar/v26i2.8102.
Jayakumar R, Chennazhi KP, Muzzarelli RAA, Tamura H, Nair SV, Selvamurugan N. Chitosan conjugated DNA nanoparticles in gene therapy. Carbohydr Polym. 2010;79:1–8. https://doi.org/10.1016/j.carbpol.2009.08.026.
Nagarwal RC, Kant S, Singh PN, Maiti P, Pandit JK. Polymeric nanoparticulate system: a potential approach for ocular drug delivery. J Control Release. 2009;136:2–13. https://doi.org/10.1016/j.jconrel.2008.12.018.
Giavasis I, Harvey LM, McNeil B. Gellan gum. Crit Rev Biotechnol. 2000;20:177–211. https://doi.org/10.1080/07388550008984169.
Picone CSF, Cunha RL. Chitosan–gellan electrostatic complexes: influence of preparation conditions and surfactant presence. Carbohydr Polym. 2013;94:695–703. https://doi.org/10.1016/j/carbpol.2013.01.092.
Ribeiro LN, Alcantara AC, Darder M, Aranda P, Araujo-Moreira FM, Ruiz-Hitzky E. Pectin-coated chitosan-LDH bionanocomposite beads as potential systems for colon-targeted drug delivery. Int J Pharm. 2014;463:1–9. https://doi.org/10.1016/j.ijpharm.2013.12.035.
Silva-Correia J, Oliveira JM, Caridade SG, Oliveira JT, Sousa RA, Mano JF, et al. Gellan gum-based hydrogels for intervertebral disc tissue-engineering applications. J Tissue Eng Regen Med. 2011;5:97–107. https://doi.org/10.1002/term.363.
Birch NP, Schiffman JD. Characterization of self-assembled polyelectrolyte complex nanoparticles formed from chitosan and pectin. Langmuir. 2014;30:3441–7. https://doi.org/10.1021/la500491c.
Kathle PK, Gautam N, Kesavan K. Tamoxifen citrate loaded chitosan-gellan nanocapsules for breast cancer therapy: development, characterisation and in-vitro cell viability study. J Microencapsul. 2018;35:292–300. https://doi.org/10.1080/02652048.2018.1477844.
Gibaldi M, Feldman S. Establishment of sink conditions in dissolution rate determinations. Theoretical considerations and application to nondisintegratin dosage forms. J Pharm Sci. 1967;56:1238–42. https://doi.org/10.1002/jps.2600561005.
Katiyar S, Pandit J, Mondal RS, Mishra AK, Chuttani K, Aqil M, et al. In situ gelling dorzolamide loaded chitosan nanoparticles for the treatment of glaucoma. Carbohydr Polym. 2014;102:117–24. https://doi.org/10.1016/j.carbpol.2013.10.079.
Hassan EE, Gallo JM. A simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strength. Pharm Res. 1990;7:491–5. https://doi.org/10.1023/a:1015812615635.
Kesavan K, Pandit JK, Kant S, Muthu MS. Positively charged microemulsions of dexamethasone: comparative effects of two cosurfactants on ocular drug delivery and bioavailability. Ther Deliv. 2013;4:1385–95. https://doi.org/10.4155/tde.13.106.
Bagre AP, Jain K, Jain NK. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: in vitro and in vivo assessment. Int J Pharm. 2013;456:31–40. https://doi.org/10.1016/j.ijpharm.2013.08.037.
Higuchi T. Mechanism of sustained-action medication. theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–9. https://doi.org/10.1002/jps.2600521210.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas N.A. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 1983;15:25-35. https://doi.org/10.1016/0378-5173(83)90064-9.
Kesavan K, Nath G, Pandit J. Preparation and in vitro antibacterial evaluation of gatifloxacin mucoadhesive gellan system. Daru. 2010;18:237–46.
Swain GP, Patel S, Gandhi J, Shah P. Development of moxifloxacin hydrochloride loaded in-situ gel for the treatment of periodontitis: In-vitro drug release study and antibacterial activity. J Oral Biol Craniofac Res 2019;9:190-200. 10.1016/j.jobcr.2019.04.001
Sharma R, Ahuja M, Kaur H. Thiolated pectin nanoparticles: preparation, characterization and ex vivo corneal permeation study. Carbohydr Polym. 2012;87:1606–10. https://doi.org/10.1016/j.carbpol.2011.09.065.
Jaiswal M, Kumar M, Pathak K. Zero order delivery of itraconazole via polymeric micelles incorporated in situ ocular gel for the management of fungal keratitis. Colloids Surf B: Biointerfaces. 2015;130:23–30. https://doi.org/10.1016/j.colsurfb.2015.03.059.
Vinardell MP, Macian M. Comparative study of the HET-CAM test and the Draize eye test for assessment of irritancy potential. Toxicol in Vitro. 1994;8:467–70. https://doi.org/10.1016/0887-2333(94)90170-8.
Rahaiee S, Hashemi M, Shojaosadati SA, Moini S, Razavi SH. Nanoparticles based on crocin loaded chitosan-alginate biopolymers: antioxidant activities, bioavailability and anticancer properties. Int J Biol Macromol. 2017;99:401–8. https://doi.org/10.1016/j.ijbiomac.2017/02/095.
Ameeduzzafar ISS, Abbas Bukhari SN, Ahmad J, Ali A. Formulation and optimization of levofloxacin loaded chitosan nanoparticle for ocular delivery: In-vitro characterization, ocular tolerance and antibacterial activity. Int J Biol Macromol. 2018;108:650–9. https://doi.org/10.1016/j.ijbiomac.2017.11.170.
Kesavan K, Kant S, Pandit JK. Therapeutic effectiveness in the treatment of experimental bacterial keratitis with ion-activated mucoadhesive hydrogel. Ocul Immunol Inflamm. 2016;24:489–92. https://doi.org/10.3109/09273948.2015.1005238.
Quinones JP, Peniche H, Peniche C. Chitosan based self-assembled nanoparticles in drug delivery. Polymers. 2018;10:235. https://doi.org/10.3390/polym10030235.
Chellat F, Tabrizian M, Dumitriu S, Chornet E, Magny P, Rivard CH, et al. In vitro and in vivo biocompatibility of chitosan-xanthan polyionic complex. J Biomed Mater Res. 2000;51:107–16. https://doi.org/10.1002/(sici)10974636(200007)51:1<107::aidjbm14>3.0.co;2-f.
Douglas KL, Tabrizian M. Effect of experimental parameters on the formation of alginate-chitosan nanoparticles and evaluation of their potential application as DNA carrier. J Biomater Sci Polym Ed. 2005;16:43–56. https://doi.org/10.1163/1568562052843339.
Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci. 1997;63:125–32. https://doi.org/10.1002/(SICI)1097-4628(19970103)63:1<125::AID-APP13>3.0.CO;2-4.
Gade S, Patel KK, Gupta C, Anjum MM, Deepika D, Agrawal AK, et al. An ex vivo evaluation of moxifloxacin nanostructured lipid carrier enriched in situ gel for transcorneal permeation on goat cornea. J Pharm Sci. 2019;108:2905–16. https://doi.org/10.1016/j.xphs.2019.04.005.
Chen PH, Kuo TY, Kuo JY, Tseng YP, Wang DM, Lai JY, et al. Novel chitosan–pectin composite membranes with enhanced strength, hydrophilicity and controllable disintegration. Carbohydr Polym. 2010;82:1236–42. https://doi.org/10.1016/j.carbpol.2010/06.057.
Prezotti FG, Cury BS, Evangelista RC. Mucoadhesive beads of gellan gum/pectin intended to controlled delivery of drugs. Carbohydr Polym. 2014;113:286–95. https://doi.org/10.1016/j.carbpol.2014.07.021.
Fialho SL, da Silva-Cunha A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin Exp Ophthalmol. 2004;32:626–32. https://doi.org/10.1111/j.1442-9071.2004.00914.x.
Kesavan K, Kant S, Singh PN, Pandit JK. Mucoadhesive chitosan-coated cationic microemulsion of dexamethasone for ocular delivery: in vitro and in vivo evaluation. Curr Eye Res. 2013;38:342–52. https://doi.org/10.3109/02713683.2012.745879.
Zhu H, Chauhan A. Effect of viscosity on tear drainage and ocular residence time. Optom Vis Sci. 2008;85:715–25. https://doi.org/10.1097/OPX.0b013e3181824dc4.
Snibson GR, Greaves JL, Soper ND, Tiffany JM, Wilson CG, Bron AJ. Ocular surface residence times of artificial tear solutions. Cornea. 1992;11:288–93. https://doi.org/10.1097/00003226-199207000-00003.
Nanjawade BK, Manvi FV, Manjappa AS. Retracted: in situ-forming hydrogels for sustained ophthalmic drug delivery. J Control Release. 2007;122:119–34. https://doi.org/10.1016/j.jconrel.2007.07.009.
Dubey V, Mohan P, Dangi JS, Kesavan K. Brinzolamide loaded chitosan-pectin mucoadhesive nanocapsules for management of glaucoma: formulation, characterization and pharmacodynamic study. Int J Biol Macromol. 2020;152:1224–32. https://doi.org/10.1016/j.ijbiomac.2019.10.219.
Acknowledgement
The authors deeply recognize the help of the Department of University Institute of Pharmacy and the SOS in Physics and Astrophysics, Pt. Ravishankar Shukla University, Raipur, Chhattisgarh, India, for carrying out the particle size specification and DSC pattern of the samples respectively. The authors acknowledge the support of Department of metallurgical engineering, National Institute of Technology, Raipur, Chhattisgarh, India for performing the XRD analysis samples. The authors were indebted to acknowledge Department of Anatomy, AIIMS, New Delhi, India for performing TEM images of samples. The authors would like to record their truthful thanks to the Department of Biotechnology, Guru Ghasidas University, Bilaspur to accomplish the antimicrobial efficacy studies. The authors were ever grateful to Orex Pharma Pvt. Ltd. (Mumbai, India) for supplying the gift sample.
Funding
Guru Ghasidas Vishwavidyalaya (A Central University), Bilaspur (C.G), Chhattisgarh, India has provided financial support in the form of a fellowship to carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohan, P., Kesavan, K. Cationic Polyelectrolyte Nanocapsules of Moxifloxacin for Microbial Keratitis Therapy: Development, Characterization, and Pharmacodynamic Study. AAPS PharmSciTech 22, 195 (2021). https://doi.org/10.1208/s12249-021-02039-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02039-1