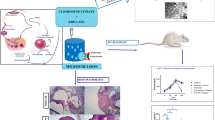
Abstract
Hirsutism is a dermatological condition that refers to the excessive growth of hair in androgen-sensitive areas in women. Recently, the enhancement of the visible signs of a hairy female has taken special concern that affected the quality of life. The present study was developed to compare the follicular targeting effect of topical spironolactone (SP) or progesterone (PG)-loaded nanostructured lipid carrier (NLC) on the management of hirsutism. Four NLC formulations were prepared using cold homogenization techniques and pharmaceutically evaluated. SP-NLC and PG-NLC topical hydrogels were prepared to explore their pharmacological effect on letrozole induced polycystic ovarian syndrome (PCOS) in rats. Inflammatory mediators, antioxidant, and hormonal parameters were assayed. Additionally, histopathological examination was carried out to confirm the successful induction of PCOS. Results confirmed that all NLC formulations have a spherical shape with particle size ranged from 225.92 ± 0.41 to 447.80 ± 0.66 nm, entrapment efficiency > 75%, and zeta potential (− 31.4 to − 36.5 mV). F1 and F3 NLCs were considered as selected formulations for SP and PG, respectively. Female Wistar rats treated with F1 formulation for 3 weeks displayed better outcomes as manifested by the measured parameters as compared to the other tested groups. A significant reduction in hair follicle diameter and density was observed after topical application of SP or PG nano-gels. Finally, the outcomes pose a strong argument that the development of topically administered SP-NLC can be explored as a promising carrier over PG-NLC for more effectual improvement in the visible sign of hirsutism.








Similar content being viewed by others
References
Hunter MH, Carek PJ. Evaluation and treatment of women with hirsutism. Am Fam Physician. 2003;67(12):2565–72.
Archer JS, Chang RJ. Hirsutism and acne in polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004;18(5):737–54.
Chazenbalk G, Chen Y-H, Heneidi S, Lee J-M, Pall M, Chen Y-DI, et al. Abnormal expression of genes involved in inflammation, lipid metabolism, and Wnt signaling in the adipose tissue of polycystic ovary syndrome. J Clin Endocrinol Metab. 2012;97(5):E765–E70.
Diamanti-Kandarakis E, Christakou CD. Insulin resistance in PCOS. Diagnosis and management of polycystic ovary syndrome: Springer; 2009. p. 35–61.
Rebar R, Judd H, Yen S, Rakoff J, Vandenberg G, Naftolin F. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest. 1976;57(5):1320–9.
Endou H, Hosoyamada M. Potassium-retaining diuretics: aldosterone antagonists. Diuretics Handb Exp Pharmacol. 1995;117:335–61.
Rathnayake D, Sinclair R. Innovative use of spironolactone as an antiandrogen in the treatment of female pattern hair loss. Dermatol Clin. 2010;28(3):611–708.
Chen W-C, Zouboulis CC. Hormones and the pilosebaceous unit. Dermatoendocrinol. 2009;1(2):81–6.
Cumming DC, Yang JC, Rebar RW, Yen SS. Treatment of hirsutism with spironolactone. Jama. 1982;247(9):1295–8.
Macias H, Hinck L. Mammary gland development. Wiley Interdiscip Rev Dev Biol. 2012;1(4):533–57.
Burkman RT Jr. The role of oral contraceptives in the treatment of hyperandrogenic disorders. Am J Med. 1995;98(1):S130–S6.
Müller R, Radtke M, Wissing S. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. 2002;242(1-2):121–8.
Knorr F, Lademann J, Patzelt A, Sterry W, Blume-Peytavi U, Vogt A. Follicular transport route–research progress and future perspectives. Eur J Pharm Biopharm. 2009;71(2):173–80.
Kaur S, et al. Nanostructure lipid carrier (NLC): the new generation of lipid nanoparticles. Asian Pac J Health Sci. 2015;2:76–93.
Nandvikar NY, Lala RR, Shinde AS. Nanostructured lipid carrier: the advanced lipid carriers. Int J Pharm Sci. 2019;10(12):5252–65.
Kurakula M, Ahmed OA, Fahmy UA, Ahmed TA. Solid lipid nanoparticles for transdermal delivery of avanafil: optimization, formulation, in-vitro and ex-vivo studies. J Liposome Res. 2016;26:288–96.
Upreti T, Senthil V. Nanostructured lipid carrier system for the treatment for skin disease—a review. JSM Nanotechnol Nanomed. 2017;5(3):1059–64.
Shekhawat PB. Preparation and evaluation of clotrimazole nanostructured lipid carrier for topical delivery. Int J Pharm Bio Sci. 2013;4:407–16.
Dong Y, Ng WK, Shen S, Kim S, Tan RB. Preparation and characterization of spironolactone nanoparticles by antisolvent precipitation. Int J Pharm. 2009;375(1-2):84–8.
Wissing S, Müller R. Solid lipid nanoparticles as carrier for sunscreens: in vitro release and in vivo skin penetration. J Control Release. 2002;81:225–33.
Csóka I, Csányi E, Zapantis G, Nagy E, Fehér-Kiss A, Horváth G, et al. In vitro and in vivo percutaneous absorption of topical dosage forms: case studies. Int J Pharm. 2005;291(1-2):11–9.
Yin-Ku L, Zih-Rou H, Rou-Zi Z, Jia-You F. Combination of calcipotriol and methotrexate in nanostructured lipid carriers for topical delivery. Int J Nanomedicine. 2010;5:117–28.
Friuli V, Bruni G, Musitelli G, Conte U, Maggi L. Influence of dissolution media and presence of alcohol on the in vitro performance of pharmaceutical products containing an insoluble drug. J Pharm Sci. 2018;107(1):507–11.
Rohman A, Nugroho A, Lukitaningsih E, Sudjadi. Application of vibrational spectroscopy in combination with chemometrics techniques for authentication of herbal medicine. Appl Spectrosc Rev. 2014;49:603–13.
Mura, et al. Characterization and evaluation of different mesoporous silica kinds as carriers for the development of effective oral dosage forms of glibenclamide. Int J Pharm. 2019;563:43–52.
Joshi B, Singh G, Rana A, Saini S, Singla V. Emulgel: a comprehensive review on the recent advances in topical drug delivery. Int Res J Pharm. 2011;2(11):66–70.
Kafali H, Iriadam M, Ozardalı I, Demir N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. 2004;35(2):103–8.
Li Q, Cai T, Huang Y, Xia X, Cole S, Cai Y. A review of the structure, preparation, and application of NLCs, PNPs, and PLNs. Nanomaterials. 2017;7(6):122.
Manjunath K, Reddy JS, Venkateswarlu V. Solid lipid nanoparticles as drug delivery systems. Methods Find Exp Clin Pharmacol. 2005;27(2):127–44.
Zakir F, Vaidya B, Goyal AK, Malik B, Vyas SP. Development and characterization of oleic acid vesicles for the topical delivery of fluconazole. Drug Deliv. 2010;17(4):238–48.
Agrawal Y, Petkar KC, Sawant KK. Development, evaluation and clinical studies of acitretin loaded nanostructured lipid carriers for topical treatment of psoriasis. Int J Pharm. 2010;401(1-2):93–102.
Paruvathanahalli SR, Jestin C. Development and evaluation of nanostructured lipid carrier-based hydrogel for topical delivery of 5-fluorouracil. Int J Nanomedicine. 2016;11:5067–77.
Chauhan I, et al. Nanostructured lipid carrier: the groundbreaking approach for transdermal drug delivery. Adv Pharm Bull. 2020;10(2):150–65.
Gaba B, Fazil M, Khan S, Ali A, Baboota S, Ali J. Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bull Fac Pharm Cairo Univ. 2015;53(2):147–59.
Jiao J. Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv Drug Deliv Rev. 2008;60:1663–73.
Hu F-Q, Jiang S-P, Du Y-Z, Yuan H, Ye Y-Q, Zeng S. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf B: Biointerfaces. 2005;45(3-4):167–73.
Lu GW, Gao P. Emulsions and microemulsions for topical and transdermal drug delivery. In: Kulkarni VS, editor. Handbook of Non-Invasive Drug Delivery Systems; 2010. p. 59–94.
Xia D, Cui F, Gan Y, Mu H, Yang M. Design of lipid matrix particles for fenofibrate: effect of polymorphism of glycerol monostearate on drug incorporation and release. J Pharm Sci. 2014;103(2):697–705.
Shah S, Joshi S, Lin S, Madan P. Preparation and characterization of spironolactone solid dispersions using hydrophilic carriers. Asian J Pharm Sci. 2012;7(1):40–9.
Kumar N, Goindi S, Saini B, Bansal G. Thermal characterization and compatibility studies of itraconazole and excipients for development of solid lipid nanoparticles. J Therm Anal Calorim. 2014;115:2375–83.
Seyfoddin A, Shaw J, Al-Kassas R. Solid lipid nanoparticles for ocular drug delivery. Drug Deliv. 2010;17:467–89.
Revathy S, Vrinda S. Kumar and Sabitha M. Omega-3 fatty acid based nanolipid formulation of atorvastatin for treating hyperlipidaemia. Adv Pharm Bull. 2019;9(2):271–80.
Yang H, Lee SY, Lee SR, Pyun B-J, Kim HJ, Lee YH, et al. Therapeutic effect of Ecklonia Cava Extract In Letrozole-induced polycystic ovary syndrome rats. Front Pharmacol. 2018;9:1325.
Caldwell A, Middleton L, Jimenez M, Desai R, McMahon A, Allan C, et al. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology. 2014;155(8):3146–59.
Choi S-H, Shapiro H, Robinson GE, Irvine J, Neuman J, Rosen B, et al. Psychological side-effects of clomiphene citrate and human menopausal gonadotrophin. J Psychosom Obstet Gynaecol. 2005;26(2):93–100.
Rezvanfar M, Rezvanfar M, Ahmadi A, Saadi HS, Baeeri M, Abdollahi M. Mechanistic links between oxidative/nitrosative stress and tumor necrosis factor alpha in letrozole-induced murine polycystic ovary: biochemical and pathological evidences for beneficial effect of pioglitazone. Hum Exp Toxicol. 2012;31(9):887–97.
Pandey V, Singh A, Singh A, Krishna A, Pandey U, Tripathi YB. Role of oxidative stress and low-grade inflammation in letrozole-induced polycystic ovary syndrome in the rat. Reprod Biol. 2016;16(1):70–7.
Christy NA, Franks AS, Cross LB. Spironolactone for hirsutism in polycystic ovary syndrome. Ann Pharmacother. 2005;39(9):1517–21.
Spritzer PM, Lisboa KO, Mattiello S, Lhullier F. Spironolactone as a single agent for long-term therapy of hirsute patients. Clin Endocrinol. 2000;52(5):587–94.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amer, R.I., Yassin, G.E., Mohamed, R.A. et al. Pharmaceutical and Pharmacological Evaluation of the Effect of Nano-Formulated Spironolactone and Progesterone on Inflammation and Hormonal Levels for Managing Hirsutism Experimentally Induced in Rats. AAPS PharmSciTech 22, 204 (2021). https://doi.org/10.1208/s12249-021-02003-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02003-z