Abstract
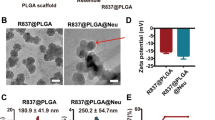
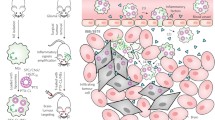
It is well known that neutrophil-mediated delivery of therapeutic agents is a promising method for treating tumors. However, owing to the limited number and limited uptake ability of neutrophils, determining a reasonable dose has become an urgent problem to be solved. Furthermore, the number of nanoparticles is far greater than the number of neutrophils at normal doses, which causes excessive nanoparticles to reach nontargeted organs or tissues, leading to serious adverse effects. To address these problems, a neutrophil-targeting delivery system (DiR-DADGC-L) based on DiR-labeled and butanedioic acid (DA)-linked 5-amino-3,5-dideoxy-D-Glycerol-D-galactonanulose-cholesterol conjugate (DADGC) was designed to improve the efficiency of hitchhiking neutrophils through the specific binding of sialic acid (SA) to L-selectin (SA-binding receptor, expressed on neutrophils). DiR-DADGC-L was prepared with favorable particle size and encapsulation efficiency (%EE) to deliver DiR into neutrophils. Subsequently, diverse doses of DiR-DADGC-L were injected intravenously into S180 tumor-bearing and cyclophosphamide-depleted (CTX-D) S180 tumor-bearing mice to evaluate the in vivo behavior of liposomes. The results verified the following: a) The content of DiR-DADGC-L in neutrophils accounts for approximately 14.5% of the content of DiR-DADGC-L in plasma, and the uptake capacity of neutrophils remains unchanged under different doses, and b) both neutrophils and the enhanced permeability and retention (EPR) effect might exert significant roles in tumor treatment. As for the neutrophil-mediated delivery system, higher doses are not necessarily appropriate, and a lower dose may achieve an unexpected effect. It will be wise to determine an optimum dose to improve delivery efficiency.









Similar content being viewed by others
References
Choi J, Kim HY, Ju EJ, Jung J, Park J, Chung HK, et al. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. 2012;33(16):4195–203.
Xinyue D, Dafeng C, Zhenjia W. Leukocyte-mediated Delivery of Nanotherapeutics in Inflammatory and Tumor Sites. Theranostics. 2017;7(3):751.
Woods J, Davis J, Smith J, Nieman D. Exercise and cellular innate immune function. Rehabilitation Oncology. 2001;19(2):34.
Mayadas TN, Cullere X, Lowell CA. The Multifaceted Functions of Neutrophils. Other. 2014;9.
Powell DR, Huttenlocher A. Neutrophils in the Tumor Microenvironment. Trends in Immunology. 2016;37:41–52.
Stefan HE, Kaufmann. Immunology's foundation: the 100-year anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff. Nature immunology. 2008.
Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–75. https://doi.org/10.1038/nri3399.
Raccosta L, Fontana R, Maggioni D, Lanterna C, Villablanca EJ, Paniccia A, et al. The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. Journal of Experimental Medicine. 2013;210(9).
Viola A, Sarukhan A, Bronte V, Molon B. The pros and cons of chemokines in tumor immunology. Trends in Immunology. 2012;33(10):496–504.
Benevides L, Fonseca DMD, Donate PB, Tiezzi DG, De Carvalho DD, De Andrade JM, et al. IL17 Promotes Mammary Tumor Progression by Changing the Behavior of Tumor Cells and Eliciting Tumorigenic Neutrophils Recruitment. Cancer Research. 2015;75:3788–99.
Fossati G, Ricevuti G, Edwards SW, Walker C, Dalton A, Rossi ML. Neutrophil infiltration into human gliomas. Acta Neuropathologica. 1999;98(4):349–54.
Moore RJ, Owens DM, Stamp G, Arnott C, Balkwill F. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nature Medicine. 1999;5(7):828–31.
Seth, Coffelt, Kelly, Kersten, Chris, Doornebal, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015.
Feng Y, Santoriello C, Mione M, Hurlstone A, Martin P, Kemp C. Live Imaging of Innate Immune Cell Sensing of Transformed Cells in Zebrafish Larvae: Parallels between Tumor Initiation and Wound Inflammation. Plos Biology. 2010;8(12):e1000562.
Xue J, Zhao Z, Zhang L, Xue L, Zhang C. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Science Foundation in China. 2017;12(3):692.
Peters AM. Just How Big is the Pulmonary Granulocyte Pool? Clinical Science. 1998;94(1):7–19.
Summers C, Rankin SM, Condliffe AM. Singh N. Chilvers ER. Neutrophil kinetics in health and disease. Trends in Immunology: Peters AM; 2010.
Fadok VA, Bratton DL, Rose DM, Pearson A, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405(6782):85–90.
Kodi S, Ravichandran. Beginnings of a Good Apoptotic Meal: The Find-Me and Eat-Me Signaling Pathways - ScienceDirect. Immunity. 2011;35(4):445–55.
Luo X, Hu L, Zheng H, Liu M, Liu X, Li C, et al. Neutrophil-mediated delivery of pixantrone-loaded liposomes decorated with poly(sialic acid)–octadecylamine conjugate for lung cancer treatment. Drug Delivery. 2018;25(1):1200–12.
Chu D, Gao J, Wang Z. Neutrophil-Mediated Delivery of Therapeutic Nanoparticles across Blood Vessel Barrier for Treatment of Inflammation and Infection. Acs Nano. 2015;9(12):11800–11.
Daruwalla J. EPR Effect. European Journal of Pharmaceutics and Biopharmaceutics. 2010.
Jain S, Raza K, Agrawal AK, Vaidya A. EPR effect and its implications in passive targeting of nanocarriers to tumors - ScienceDirect. Nanotechnology Applications for Cancer Chemotherapy. 2021:31–40.
Arbonés ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1(4):247–60.
Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860.
Bargatze RF, Kurk S, Butcher EC, Jutila MA. Neutrophils roll on adherent neutrophils bound to cytokine-induced endothelial cells via L-selectin on the rolling cells. Journal of Experimental Medicine. 1994;180(5):1785–92.
English D, Andersen BR. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. Journal of Immunological Methods. 1974;5(3):249–52.
Van Vlasselaer P. Methods for enriching specific cell-types by density gradient centrifugation. US. 1998.
Sialic acid-conjugate modified liposomes targeting neutrophils for improved tumour therapy. Biomaterials ence. 2020;8.
She Z, Zhang T, Wang X, Li X, Deng Y. The anticancer efficacy of pixantrone-loaded liposomes decorated with sialic acid–octadecylamine conjugate. Biomaterials. 2014;35(19):5216–25.
Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. Journal of Immunology. 1993;151(5):2399–408.
Hahm E, Li J, Kim K, Huh S, Rogelj S, Cho J. Extracellular protein disulfide isomerase regulates ligand-binding activity of αMβ2 integrin and neutrophil recruitment during vascular inflammation. Blood. 2013;121(19):3789–800.
Sumagin R, Prizant H, Lomakina E, Waugh RE, Sarelius IH, Sumagin R, et al. LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J. Immunol. 185, 7057-7066. Journal of Immunology. 2010;185(11):7057–66.
Wang Z, Jing L, Cho J, Malik AB. Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils. Nature Nanotechnology. .
Zhou S, Zhang T, Peng B, Luo X, Liu X, Hu L, et al. Targeted delivery of epirubicin to tumor-associated macrophages by sialic acid-cholesterol conjugate modified liposomes with improved antitumor activity. International Journal of Pharmaceutics. 2017;523(1):203–16.
Ghio AJ, Kennedy TP, Hatch GE, Tepper JS. Reduction of neutrophil influx diminishes lung injury and mortality following phosgene inhalation. Journal of Applied Physiology. 1991;71(2):657–65.
Timmermans K, Wal… SEVD. IL-1β processing in mechanical ventilation-induced inflammation is dependent on neutrophil factors rather than caspase-1. Intensive Care Medicine Experimental. 2013;1.
Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing Liposomes for Delivery of Chemotherapeutic Agents to Solid Tumors. Pharmacological Reviews. 1999;51(4):691–743.
Chibowski E, Szcze? A. Zeta potential and surface charge of DPPC and DOPC liposomes in the presence of PLC enzyme. Adsorption. 2016;22(4-6):755-765.
Dafeng C. Qi, Zhao, Jian, Yu, et al. Advanced Healthcare Materials: Nanoparticle Targeting of Neutrophils for Improved Cancer Immunotherapy; 2016.
Mononuclear Phagocyte System (MPS): Springer Berlin Heidelberg; 2016.
Allen TM. Ligand-targeted therapeutics in anticancer therapy. Angewandte Chemie International Edition. 2002;2:705–63.
Allen TM, Cullis PR. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv Drug Deliv Rev. 2012;65(1):36–48.
Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery. .
Tang J, Zhang L, Liu Y, Zhang Q, Qin Y, Yin Y, et al. Synergistic targeted delivery of payload into tumor cells by dual-ligand liposomes co-modified with cholesterol anchored transferrin and TAT. International Journal of Pharmaceutics. 2013;454(1):31–40.
Epstein H, Afergan E, Moise T, Richter Y, Rudich Y, Golomb G. Number-concentration of nanoparticles in liposomal and polymeric multiparticulate preparations: Empirical and calculation methods. Biomaterials. 2006;27(4):651–9.
Lis LJ, Mcalister M, Fuller N, Rand RP, Parsegian VA. Interaction between Neutral Phospholipid Bilayer Membranes. Biophysical Journal. 1982;37(3):657–65.
Müller-Landau F, Cadenhead DA. Molecular packing in steroid-lecithin monolayers, part II: Mixed films of cholesterol with dipalmitoylphosphatidylcholine and tetradecanoic acid. Chemistry and Physics of Lipids. 1979.
A good practice guide to the administration of substances and removal of blood, including routes and volumes. Journal of Applied Toxicology Jat. 2001;21(1):15.
Morris MR, Doull IJM, Dewitt S, Hallett MB. Reduced iC3b-mediated phagocytotic capacity of pulmonary neutrophils in cystic fibrosis. Clinical & Experimental Immunology. 2010;142(1):68–75.
Acknowledgments
This research was supported by the National Natural Science Foundation of China [No. 81973271].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 435 kb)
Rights and permissions
About this article
Cite this article
Liu, M., Li, C., NaYan et al. Influence of Dose on Neutrophil-Mediated Delivery of Nanoparticles for Tumor-Targeting Therapy Strategies. AAPS PharmSciTech 22, 89 (2021). https://doi.org/10.1208/s12249-021-01959-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-01959-2