Abstract
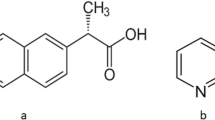
Poor physicomechanical properties and limited aqueous solubility restrict the bioavailability of aceclofenac when given orally. To improve its above properties, aceclofenac (ACE) was cocrystallized with dimethyl urea (DMU) in 1:2 molar ratio by dry and solvent assisted grinding. The cocrystals were characterized by ATR-FTIR, DSC, and PXRD, and their surface morphology was studied by SEM. There was enhancement in intrinsic dissolution rate (IDR) (~eight- and ~fivefold in cocrystals prepared by solvent assisted grinding (SAG) and solid state grinding (SSG), respectively, in 0.1 N HCl, pH 1.2) and similarly (~3.42-fold and ~1.20-fold in phosphate buffer, pH 7.4) as compared to pure drug. Additionally, mechanical properties were assessed by tabletability curves. The tensile strength of ACE was < 1 MPa in contrast to the cocrystal tensile strength (3.5 MPa) which was ~1.98 times higher at 6000 psi. The tablet formulation of cocrystal by direct compression displayed enhanced dissolution profile (~36% in 0.1 N HCl, pH 1.2, and ~100% in phosphate buffer, pH 7.4) in comparison to physical mixture (~ 30% and ~ 80%) and ACE (~18% and ~50%) after 60 min, respectively. Stability studies of cocrystal tablets for 3 months indicated a stable formulation. Pharmacokinetic studies were performed by using rabbit model. The AUC0-∞ (37.87±1.3 μgh/ml) and Cmax (6.94±2.94 μg/ml) of the selected cocrystal C1 prepared by SAG were significantly enhanced (p < 0.05) and were ~3.43 and ~1.63-fold higher than that of ACE. In conclusion, new cocrystal of ACE-DMU was successfully prepared with improved tabletability, in vitro and in vivo properties.











Similar content being viewed by others
References
Karagianni A, Malamatari M, Kachrimanis K. Pharmaceutical cocrystals: new solid phase modification approaches for the formulation of APIs. Pharmaceutics. 2018;10(1):18.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol. 2010;62(11):1607–21.
Aakery CB, Salmon DJ. Building co-crystals with molecular sense and supramolecular sensibility. 2005.
Childs SL, Zaworotko MJ. The reemergence of cocrystals: the crystal clear writing is on the wall introduction to virtual special issue on pharmaceutical cocrystals: ACS Publications; 2009.
Henck J-O, Byrn SR. Designing a molecular delivery system within a preclinical timeframe. Drug Discov Today. 2007;12(5-6):189–99.
Friščić T, Jones W. Benefits of cocrystallisation in pharmaceutical materials science: an update. J Pharm Pharmacol. 2010;62(11):1547–59.
Sayed E, Haj-Ahmad R, Ruparelia K, Arshad M, Chang M-W, Ahmad Z. Porous inorganic drug delivery systems—a review. AAPS PharmSciTech. 2017;18(5):1507–25.
Tong HH, Chow AS, Chan H, Chow AH, Wan YK, Williams ID, et al. Process-induced phase transformation of berberine chloride hydrates. J Pharm Sci. 2010;99(4):1942–54.
Datta S, Grant DJ. Crystal structures of drugs: advances in determination, prediction and engineering. Nat Rev Drug Discov. 2004;3(1):42–57.
Blagden N, de Matas M, Gavan PT, York P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv Drug Deliv Rev. 2007;59(7):617–30.
Desiraju GR. Chemistry beyond the molecule. Nature. 2001;412(6845):397–400.
Desiraju GR. Crystal engineering: a brief overview. J Chem Sci. 2010;122(5):667–75.
Schultheiss N, Newman A. Pharmaceutical cocrystals and their physicochemical properties. Cryst Growth Des. 2009;9(6):2950–67.
Shan N, Perry ML, Weyna DR, Zaworotko MJ. Impact of pharmaceutical cocrystals: the effects on drug pharmacokinetics. Expert Opin Drug Metab Toxicol. 2014;10(9):1255–71.
Miroshnyk I, Mirza S, Sandler N. Pharmaceutical co-crystals–an opportunity for drug product enhancement. Expert Opin Drug Deliv. 2009;6(4):333–41.
Abbas N, Latif S, Afzal H, Arshad MS, Hussain A, Sadeeqa S, et al. Simultaneously improving mechanical, formulation, and in vivo performance of naproxen by co-crystallization. AAPS PharmSciTech. 2018;19(7):3249–57.
Brittain HG. Cocrystal systems of pharmaceutical interest: 2010. Cryst Growth Des. 2012;12(2):1046–54.
Arora KK, Zaworotko MJ. Pharmaceutical co-crystals: a new opportunity in pharmaceutical science for a long-known but little-studied class of compounds. Polymorphism in Pharmaceutical Solids: CRC Press. 2018:294–329.
Shan N, Zaworotko MJ. The role of cocrystals in pharmaceutical science. Drug Discov Today. 2008;13(9-10):440–6.
Sharma G, Saini MK, Thakur K, Kapil N, Garg NK, Raza K, et al. Aceclofenac cocrystal nanoliposomes for rheumatoid arthritis with better dermatokinetic attributes: a preclinical study. Nanomedicine. 2017;12(6):615–38.
Usha AN, Mutalik S, Reddy MS, Ranjith AK, Kushtagi P, Udupa N. Preparation and, in vitro, preclinical and clinical studies of aceclofenac spherical agglomerates. Eur J Pharm Biopharm. 2008;70(2):674–83.
Vadher AH, Parikh JR, Parikh RH, Solanki AB. Preparation and characterization of co-grinded mixtures of aceclofenac and Neusilin US 2 for dissolution enhancement of aceclofenac. AAPS PharmSciTech. 2009;10(2):606–14.
Goud NR, Suresh K, Nangia A. Solubility and stability advantage of aceclofenac salts. Cryst Growth Des. 2013;13(4):1590–601.
Wouters J, Rome S, Quéré L. Monographs of most frequent co-crystal formers. Pharmaceutical salts and co-crystals: Royal Society of Chemistry London. 2011:338–82.
Seaton CC, Parkin A. Making benzamide cocrystals with benzoic acids: the influence of chemical structure. Cryst Growth Des. 2011;11(5):1502–11.
Cysewski P, Przybyłek M, Ziółkowska D, Mroczyńska K. Exploring the cocrystallization potential of urea and benzamide. J Mol Model. 2016;22(5):103.
Schlottmann U, Stock B, Chemicals OH. SIDS initial assessment report For SIAM 17. 2005.
Baaklini G, Dupray V, Coquerel G. Inhibition of the spontaneous polymorphic transition of pyrazinamide γ form at room temperature by co-spray drying with 1, 3-dimethylurea. Int J Pharm. 2015;479(1):163–70.
Moffat AC, Osselton MD, Widdop B, Watts J. Clarke’s analysis of drugs and poisons: Pharmaceutical press London; 2011.
Chow SF, Chen M, Shi L, Chow AH, Sun CC. Simultaneously improving the mechanical properties, dissolution performance, and hygroscopicity of ibuprofen and flurbiprofen by cocrystallization with nicotinamide. Pharm Res. 2012;29(7):1854–65.
Fell J, Newton J. Determination of tablet strength by the diametral-compression test. J Pharm Sci. 1970;59(5):688–91.
Lucas TI, Bishara RH, Seevers RH. A stability program for the distribution of drug products. Pharm Technol. 2004;28:68–73.
Narayan R, Pednekar A, Bhuyan D, Gowda C, Koteshwara K, Nayak UY. A top-down technique to improve the solubility and bioavailability of aceclofenac: in vitro and in vivo studies. Int J Nanomedicine. 2017;12:4921–35.
Jung MS, Kim JS, Kim MS, Alhalaweh A, Cho W, Hwang SJ, et al. Bioavailability of indomethacin-saccharin cocrystals. J Pharm Pharmacol. 2010;62(11):1560–8.
Naz A, Beg AE, Ahmed KZ, Ali H, Naz S, Zafar F. Pharmacokinetics study of aceclofenac in pakistani population and effects of sucralfate co-administration on bioavailability of aceclofenac. J Appl Res. 2011;11(1).
Zhang Y, Huo M, Zhou J, Xie S. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Prog Biomed. 2010;99(3):306–14.
Kumar S, Gupta A, Prasad R, Singh S. Novel aceclofenac cocrystals with l-cystine: virtual coformer screening, mechanochemical synthesis, and physicochemical investigations. Curr Drug Deliv. 2020;17.
Weyna DR, Shattock T, Vishweshwar P, Zaworotko MJ. Synthesis and structural characterization of cocrystals and pharmaceutical cocrystals: mechanochemistry vs slow evaporation from solution. Cryst Growth Des. 2009;9(2):1106–23.
Jones W, Motherwell WS, Trask AV. Pharmaceutical cocrystals: an emerging approach to physical property enhancement. MRS Bull. 2006;31(11):875–9.
Sun S, Zhang H, Xu J, Wang S, Wang H, Yu Z, et al. The competition between cocrystallization and separated crystallization based on crystallization from solution. J Appl Crystallogr. 2019;52(4).
Steed JW. The role of co-crystals in pharmaceutical design. Trends Pharmacol Sci. 2013;34(3):185–93.
Padrela L, de Azevedo EG, Velaga SP. Powder X-ray diffraction method for the quantification of cocrystals in the crystallization mixture. Drug Dev Ind Pharm. 2012;38(8):923–9.
Katritzky AR, Jain R, Lomaka A, Petrukhin R, Maran U, Karelson M. Perspective on the relationship between melting points and chemical structure. Cryst Growth Des. 2001;1(4):261–5.
Simperler A, Watt SW, Bonnet PA, Jones W, Motherwell WS. Correlation of melting points of inositols with hydrogen bonding patterns. Cryst Eng Comm. 2006;8(8):589–600.
Gao Y, Gao J, Liu Z, Kan H, Zu H, Sun W, et al. Coformer selection based on degradation pathway of drugs: a case study of adefovir dipivoxil–saccharin and adefovir dipivoxil–nicotinamide cocrystals. Int J Pharm. 2012;438(1-2):327–35.
Haleblian JK. Characterization of habits and crystalline modification of solids and their pharmaceutical applications. J Pharm Sci. 1975;64(8):1269–88.
Qiao N, Li M, Schlindwein W, Malek N, Davies A, Trappitt G. Pharmaceutical cocrystals: an overview. Int J Pharm. 2011;419(1-2):1–11.
Ren S, Liu M, Hong C, Li G, Sun J, Wang J, et al. The effects of pH, surfactant, ion concentration, coformer, and molecular arrangement on the solubility behavior of myricetin cocrystals. Acta Pharm Sin B. 2019;9(1):59–73.
Latif S, Ijaz QA, Hameed M, Fatima K, Hussain A, Arshad MS, et al. Improvement of physico-mechanical and pharmacokinetic attributes of naproxen by cocrystallization with L-alanine. J Drug Deliv Sci Technol. 2020;102236.
Chang S-Y, Sun CC. Superior plasticity and tabletability of theophylline monohydrate. Mol Pharm. 2017;14(6):2047–55.
Chattoraj S, Shi L, Sun CC. Understanding the relationship between crystal structure, plasticity and compaction behaviour of theophylline, methyl gallate, and their 1: 1 co-crystal. Cryst Eng Comm. 2010;12(8):2466–72.
Acknowledgements
We would like to pay special thanks to Mr. Mohammad Moqeet Khan, from University of Veterinary and Animal Sciences, for his cooperation and help in pharmacokinetic studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Afzal, H., Abbas, N., Hussain, A. et al. Physicomechanical, stability, and pharmacokinetic evaluation of aceclofenac dimethyl urea cocrystals. AAPS PharmSciTech 22, 68 (2021). https://doi.org/10.1208/s12249-021-01938-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-01938-7