Abstract
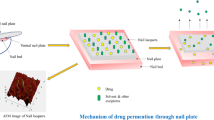
Onychomycosis is considered a stubborn nail fungal infection that does not respond to conventional topical antifungal treatments. This study aimed to develop and characterize novel solid lipid nanoparticles (SLNs) formulae containing terbinafine HCl (TFH) and loaded with different nail penetration enhancers (nPEs). Three (nPEs) N-acetyl-L-cysteine, thioglycolic acid, and thiourea were used. Characterization of the prepared formulae was done regarding particle size, zeta potential, polydispersity index (PDI), entrapment efficiency (EE%), physical stability, in vitro release study, infrared (FT-IR), and their morphological structures. The selected formulae and the marketed cream Lamifen® were compared in terms of their antifungal activity against Trichophyton rubrum as well as their nail hydration and their drug uptake by the nail clippers. Thiourea was the nPE of choice; formulae (N2 and N8), with thiourea, were considered the optimum TFH SLNs containing nPEs. They were selected for their optimum particle size of 426.3 ± 10.18 and 450.8 ± 11.45 nm as well as their highest EE% of 89.76 ± 1.25 and 90.35 ± 1.33, respectively. The in vitro microbiological screening of the antifungal activity of these two formulae showed significantly larger zones of inhibition in comparison with the marketed product. The ex vivo screening of the drug uptake of the two selected formulae was significantly higher than that of the marketed product. The nPE formulae present a very promising option as they showed optimum physicochemical characterization with high antifungal activity and high drug uptake as well as good nail hydration effect.










Similar content being viewed by others
References
Hall M, Monka C, Krupp P, OˊSulllivan D. Safety of oral terbinafine: results of a postmarketing surveillance study in 25,884 patients. Arch Dermatol. 1997;133:1213–9.
Lim EH, Kim HR, Park YO, Lee Y, Seo HJ, Kim CD, et al. Toenail onychomycosis treated with a fractional carbon-dioxide laser and topical antifungal cream. J Am Acad Dermatol. 2014;70:918–23. https://doi.org/10.1016/j.jaad.2014.01.893.
Durden F, Elewski B. Fungal infections in HIV-infected patients. Semin Cutan Med Surg. 1997;16:200–12.
Gupta AK, Cooper EA, Ryder JE, Nicol KA, Chow M, Chaudhry MM. Optimal management of fungal infections of the skin, hair and nails. Am J Clin Dermatol. 2004;5(4):225–37. https://doi.org/10.2165/00128071-200405040-00003.
Kuvandik G, Cetin M, Genctoy G, et al. The prevalence, epidemiology and risk factors for onychomycosis in hemodialysis patients. BMC Infect Dis. 2007;7:102. https://doi.org/10.1186/1471-2334-7-102.
Szepietowski JC, Salomon J. Do fungi play a role in psoriatic nails. Mycoses. 2007;50(6):437–42. https://doi.org/10.1111/j.1439-0507.2007.01405.x.
Shanbhag PP, Jani U. Drug delivery through nails: present and future. New Horizons Transl Med. 2017;3:252–63. https://doi.org/10.1016/J.NHTM.2017.01.002.
Shivakumar HN, Juluri A, Desai BG, Murthy SN. Ungual and transungual drug delivery. Drug Dev Ind Pharm. 2012;38(8):901–11. https://doi.org/10.3109/03639045.2011.637931.
Baran R, Kaoukhov A. Topical antifungal drugs for the treatment of onychomycosis: an overview of current strategies for monotherapy and combination therapy. J Eur Acad Dermatol Venereol. 2005;19:21–9. https://doi.org/10.1111/j.1468-3083.2004.00988.x.
Gupta M, Goyal AK, Paliwal SR, Paliwal R, Mishra N, Vaidya B, et al. Development and characterization of effective topical liposomal system for localized treatment of cutaneous candidiasis. J Liposome Res. 2010;20(4):341–50. https://doi.org/10.3109/08982101003596125.
Hafeez F, Hui X, Selner M, Rosenthal B, Maibach H. Ciclopirox delivery into the human nail plate using novel lipid diffusion enhancers. Drug Dev Ind Pharm. 2014;40(6):838–44. https://doi.org/10.3109/03639045.2013.788016.
Miron D, Cornelio R, Troleis J, Mariath J, Zimmer AR, Mayorga P, et al. Influence of penetration enhancers and molecular weight in antifungals permeation through bovine hoof membranes and prediction of efficiency in human nails. Eur J Pharm Sci. 2014;51:20–5. https://doi.org/10.1016/j.ejps.2013.08.032.
Gunt HB, Kasting GB. Effect of hydration on the permeation of ketoconazole through human nail plate in vitro. Eur J Pharm Sci. 2007;32(4–5):254–60. https://doi.org/10.1016/j.ejps.2007.07.009.
Akhtar N, Sharma H, Pathak K. Onychomycosis: potential of nail lacquers in transungual delivery of antifungals. Hindawi Publishing Corporation. Scientifica 2016;2016:1–12. Article ID 1387936, 12 pages, 2016. https://doi.org/10.1155/2016/1387936.
Cutrín-Gómez E, Anguiano-Igea S, Delgado-Charro MB, Gómez-Amoza JL, Otero-Espinar FJ. Effect of penetration enhancers on drug nail permeability from cyclodextrin/poloxamer-soluble polypseudorotaxane-based nail lacquers. Pharmaceutics. 2018;10(4):273. https://doi.org/10.3390/pharmaceutics10040273.
Elsherif NI, Shamma RN, Abdelbary G. Terbinafine hydrochloride trans-ungual delivery via nanovesicular systems: in vitro characterization and ex vivo evaluation. AAPS Pharm Sci Tech. 2017;18(2):551–62. https://doi.org/10.1208/s12249-016-0528-9.
Tayel SA, El-nabarawi MA, Tadros MI, Abd-elsalam WH. Positively charged polymeric nanoparticle reservoirs of terbinafine hydrochloride: preclinical implications for controlled drug delivery in the aqueous humor of rabbits. AAPS Pharm Sci Tech. 2013;14(2):782–93. https://doi.org/10.1208/s12249-013-9964-y.
Abobakr FE, Fayez SM, Elwazzan VS, Sakran W. Formulation and optimozation of terbinafine HCl solid lipid nanopaticles for topical antifunfal activity. Int J Pharm Pharm Sci. 2019;11(12):16–25. https://doi.org/10.22159/ijpps.2019v11i12.35560.
Palliyil B, Lebo DB, Patel PR. A preformulation strategy for the selection of penetration enhancers for a transungual formulation. AAPS Pharm Sci Tech. 2013;14(2):682–91. https://doi.org/10.1208/s12249-013-9954-0.
Mora-Huertas CE, Fessi H, Elaissari A. Polymer-based nanocapsules for drug delivery. Int J Pharm. 2010;385:113–42. https://doi.org/10.1016/j.ijpharm.2009.10.018.
Amin MM, Sheta NM, Abd El Gawad NA, Badawi AA. Preparation and characterization of benzophenone-3 loaded polymeric nanoparticles of lactide-co-caprolactone as drug carriers. J Pharm Res Opin. 2012;2(2):28–41.
Vitorino C, Carvalho FA, Almeida AJ, Sousa JJ, Pais AA. The size of solid lipid nanoparticles: an interpretation from experimental design. Colloids Surf B: Biointerfaces. 2011;84:117–30. https://doi.org/10.1016/j.colsurfb.2010.12.024.
Patel KK, Marya BH, Kakhanis VV. Spectrophotometric determination and validation for terbinafine hydrochloride in pure and in tablet dosage form. Scholars Res Library. 2012;4(4):1119–22.
Montenegro L, Carbone C, Puglisi G. Vehicle effects on in-vitro release and skin permeation of octylmethoxycinnamate from microemulsions. Int J Pharm. 2011;405(1–2):162–8. https://doi.org/10.1016/j.ijpharm.2010.11.036.
Gaba B, Fazil M, Khan S, Ali A, Baboota S, Ali J. Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bull Fac Pharm Cairo Univ. 2015;53:147–59.
US Pharmacopeia 42 and National Formulary 37. 2019;vol 2:p.4233.
Soma D, Attari Z, Reddy MS, Damodaram A. Solid lipid nanoparticles of irbesartan: preparation, characterization, optimization, and pharmacokinetic studies. Braz J Pharm Sci. 2017;1:1–10. https://doi.org/10.1590/s2175-97902017000115012.
Yue X, Li Q, Wang H, Sun Y, Wang A, Zhang Q, et al. An ultrastructural study of Trichophyton rubrum induced onychomycosis. BMC Infect Dis. 2015;15:1–8. https://doi.org/10.1186/s12879-015-1240-1.
Takasuka T. Amino acid- or protein-dependent growth of Trichophyton mentagrophytes and Trichophyton rubrum. FEMS Immunol Med Microbiol. 2000;29(4):241–5. https://doi.org/10.1111/j.1574-695X.2000.tb01529.x.
Barot BS, Parejiya PB, Patel HK, Gohel MC, Shelat PK. Microemulsion-based gel of terbinafine for the treatment of onychomycosis: optimization of formulation using D-optimal design. AAPS Pharm Sci Tech. 2012;13(1):184–92. https://doi.org/10.1208/s12249-011-9742-7.
Sahoo S, Pani NR, Sahoo SK. Effect of microemulsion in topical sertaconazole hydrogel: in vitro and in vivo study. Drug Deliv. 2016;23(1):338–45. https://doi.org/10.3109/10717544.2014.914601.
Devi M, Kumar SM, Mahadevan N. Amphotericin-B loaded vesicular systems for the treatment of topical fungal infection. Int J Recent Adv Pharm Res. 2011;4:37–46.
Shahin M, Abdel Hady S, Hammad M, Mortada N. Novel jojoba oil-based emulsion gel formulations for clotrimazole delivery. AAPS Pharm Sci Tech. 2011;12(1):239–47. https://doi.org/10.1208/s12249-011-9583-4.
Verma S, Bhardwaj A, Vij M, Bajpai P, Goutam N, Kumar L. Oleic acid vesicles: a new approach for topical delivery of antifungal agent. Artif Cells Nanomed Biotechnol. 2014;42(2):95–101. https://doi.org/10.3109/21691401.2013.794351.
Pal P, Thakur RS, Ray S, Mazumder B. Design and development of a safer non-invasive transungual drug delivery system for topical treatment of onychomycosis. Drug Dev Ind Pharm. 2015;41:1095–9.
Chouhan P, Saini TR. Hydration of nail plate: a novel screening model for transungual drug permeation enhancers. Int J Pharm. 2012;436(1–2):179–82. https://doi.org/10.1016/j.ijpharm.2012.06.020.
Wang Y, Pang L, Wu M, Ou N. A validated LC-MS/MS method for determination of sertaconazole nitrate in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(31):4047–50. https://doi.org/10.1016/j.jchromb.2009.09.032.
Murdan S, Milcovich G, Goriparthi GS. An assessment of the human nail plate pH. Skin Pharmacol Physiol. 2011;24(4):175–81. https://doi.org/10.1159/000324055.
Iizhar SA, Syed IA, Satar R, Ansari SA. In vitro assessment of pharmaceutical potential of ethosomes entrapped with terbinafine hydrochloride. J Adv Res. 2016;7(3):453–61. https://doi.org/10.1016/j.jare.2016.03.003.
Taplin D, Blank H. Microscopic morphology of Trichophyton rubrum. J Invest Dermatol. 1961;37:523–8.
Bseiso EA, Nasr M, Sammour OA, Abd El Gawad NA. Novel nail penetration enhancer containing vesicles “nPEVs” for treatment of onychomycosis. Drug Deliv. 2016;23:2813–9. https://doi.org/10.3109/10717544.2015.1099059.
Choudhari Y, Kulthe S, Inamdar N, Shirolikar S, Borde L, Mourya V. Combination of low and high molecular weight chitosans for the preparation of nanoparticles: a novel approach towards sustained drug delivery. J Nanopharm Drug Deliv. 2014;1:376–87. https://doi.org/10.1166/jnd.2013.1031.
Shalaby KS, Soliman ME, Casettari L, Bonacucina G, Cespi M, Palmieri GF, et al. Determination of factors controlling the particle size and entrapment efficiency of noscapine in PEG/PLA nanoparticles using artificial neural networks. Int J Nanomedicine. 2014;9:4953–64. https://doi.org/10.2147/IJN.S68737.
Manconi M, Caddeo C, Sinico C, Valenti D, Mostallino MC, Lampis S, et al. Penetration enhancer-containing vesicles: composition dependence of structural features and skin penetration ability. Eur J Pharm Biopharm. 2012;82:352–9.
Diniyanti SN, Werawatganone P, Muangsiri W. Preparation of solid lipid nanoparticles containing mangosteen pericarp extract. Asian J Pharm Clin Res. 2018;11:80–4.
Ekambaram P, Abdul Hasan Sathali A. Formulation and evaluation of solid lipid nanoparticles of ramipril. J Young Pharm. 2011;3:216–20.
Hernandez JRV, Goymann CCM. Novel nanoparticulate carrier system based on carnauba wax and decyloleate for the dispersion of inorganic sunscreens in aqueous media. Eur J Pharm Biopharm. 2005;60:113–22.
Gardouh AR, Gad S, Ghonaim HM, Ghorab MM. Design and characterization of glycerylmonostearate solid lipid nanoparticles prepared by high shear homogenization. BJPR. 2013;3:326–46.
Sharma A, Suhas R, Chandan S, Gowda DC. Novel urea and thiourea derivatives of thiazole-glutamic acid conjugate as potential inhibitors of microbes and fungi. Bioorg Khim. 2013;39(6):736–44.
Mitta M, Madhava K. Synthesis, spectral characterization and anti bacterial activity of novel thiourea analogues. Int J Curr Microbiol App Sci. 2015;4(5):900–9.
Author information
Authors and Affiliations
Contributions
The research idea was set with guidance of Prof. Dr. Wedad Sakran and Dr. Vivian S. Elwazzan. The research work and manuscript preparation were done by Fatma E. Abobakr; the research work was guided by Dr. Sahar M. Fayez, calculations and data interpretation was supported by Dr. Vivian S. Elwazzan, critical revision of the manuscript was done by Dr. Sahar M. Fayez and Dr. Vivian S. Elwazzan, and the final version of the manuscript was thoroughly revised by Dr. Vivian S. Elwazzan and finally approved by Prof. Dr. Wedad. Sakran.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abobakr, F.E., Fayez, S.M., Elwazzan, V.S. et al. Effect of Different Nail Penetration Enhancers in Solid Lipid Nanoparticles Containing Terbinafine Hydrochloride for Treatment of Onychomycosis. AAPS PharmSciTech 22, 33 (2021). https://doi.org/10.1208/s12249-020-01893-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01893-9