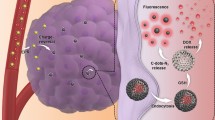
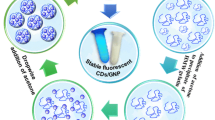
Abstract
This study reports the generation of novel, aqueous-dispersible plunoric-CD nanoconjugates encapsulating doxorubicin (Dox). The fluorescent CD were conjugated with plunoric F127 to form biocompatible delivery matrix and were further loaded with fluorescent Dox molecule. The resulting particles were analyzed for multiplexed bioimaging and targeted drug delivery. Physicochemical and optical characterization demonstrated discrete fluorescence from CD (blue emission) and Dox (orange emission) counterparts. In vitro drug release profile signifies higher and rapid release of Dox from Dox@Plu-CD under acidic conditions compared to physiological pH. Thus, the acid liable Dox@Plu-CD linkage can easily break in the cytosol of tumor cells because of low pH compared to normal cells thus conferring minimal damage to healthy cells. Moreover, results form in vitro cell viability assay suggest the cyto-compatibility of Plu-CD delivery matrix to HEK293 and HeLa cell lines. However, Dox@Plu-CD induced cell death and morphological alterations in HeLa cell lines, signifying pH-responsive effect of the prepared complex. Confocal imaging signified that Dox@Plu-CD effectively penetrates HeLa cells, and the released Dox binds to the cell nucleus and induces oxidative stress. The prepared Dox@Plu-CD thus behaved as efficient fluorescent probes allowing multiplexed bioimaging (blue and orange) of HeLa cells along with improved therapeutic potential.
Graphical abstract







Similar content being viewed by others
Abbreviations
- Dox:
-
Doxorubicin
- Plu:
-
Plunoric
- CD:
-
Carbon dots
- ROS:
-
Reactive oxygen species
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- FBS:
-
Fetal bovine serum
- DLS:
-
Dynamic light scattering
- NCCS:
-
National Centre for Cell Science
- Rh :
-
Hydrodynamic radius
- PEG:
-
Polyethylene glycol
- PBS:
-
Phosphate-buffered saline
- DCFDA:
-
2′,7′-Dichlorofluorescein-diacetate
- RPMI:
-
Roswell Park Memorial Institute Medium
References
Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. https://doi.org/10.1124/pr.56.2.6.
Pommier Y, Leteurtre Y, Fesen MR, Fujimori A, Bertrand R, Solary E, et al. Cellular determinants of sensitivity and resistance to DNA topoisomerase inhibitors. Cancer Investig. 1994;12:530–42. https://doi.org/10.3109/07357909409021413.
Upadhayay KK, Bhatt AN, Mishra AK, Dwarakanath BS, Jain S, Schatz C, Le Meins JF, Farooque A, Chandraiah G, Jain AK, Misra A, Lecommandoux S. The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly (γ-benzyl L-glutamate)-b-hyaluronanpolymersomes. Biomaterials. 2010; 31: 2882–2892. https://doi.org/10.1016/j.biomaterials.2009.12.043.
Mohan P, Rapoport N. Doxorubicin as a molecular nanotheranostic agent: effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking. Mol Pharm. 2010;7(6):1959–73. https://doi.org/10.1021/mp100269f.
Lankelma J, Dekker H, Luque FR, Luykx S, Hoekman K, der Valk van P, et al. Doxorubicin gradients in human breast cancer. Clin Cancer Res. 1999;5:1703–1707.
Devy L, de Groot FMH, Blacher S, Hajitou A, Beusker PH, Scheeren HW, Foidart JM, Noel A. Plasmin-activated doxorubicin prodrugs containing a spacer reduce tumor growth and angiogenesis without systemic toxicity. FASEB J 2004; 18: 565–567. https://doi.org/10.1096/fj.03-0462fje.
Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology. 2010;115:155–62. https://doi.org/10.1159/000265166.
Shaikh AY, Shih JA. Chemotherapy-induced cardiotoxicity. Curr Heart Fail Rep. 2012;9(2):117–27. https://doi.org/10.1007/s11897-012-0083-y.
Hruby M, Konak C, Ulbrich K. Polymeric micellar pH-sensitive drug delivery system for doxorubicin. J Control Release. 2005;103:137–48. https://doi.org/10.1016/j.jconrel.2004.11.017.
Lee Y, Park SY, Mok H, Park TG. Synthesis, characterization, antitumor activity of pluronic mimicking copolymer micelles conjugated with doxorubicin via acid-cleavable linkage. Bioconjug Chem. 2008;19:525–31. https://doi.org/10.1021/bc700382z.
Yoo HS, Park TG. Biodegradable polymeric micelles composed of doxorubicin conjugated PLGA–PEG block copolymer. J Control Release. 2001;70:63–70. https://doi.org/10.1016/S0168-3659(00)00340-0.
Lacko AG, Sabnis NA, Nagarajan B, McConathy WJ. HDL as a drug and nucleic acid delivery vehicle. Front Pharmacol. 2015;6. https://doi.org/10.3389/fphar.2015.00247.
Wang F, Wang Y, Dou S, Xiong M, Sun T, Wang J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano. 2011:53679–92. https://doi.org/10.1021/nn200007z.
Kabanov AV, Batrakova EV, Alakhov VY. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release. 2002;82(2):189–212. https://doi.org/10.1016/S0168-3659(02)00009-3.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394–424.
Sarnath D, Khanna A. Current status of cancer burden: global and Indian scenario. J Biomed Res. 2014;1(1):1–5.
Chen N, He Y, Su Y, Li X, Huang Q, Wang H, et al. The cytotoxicity of cadmium-based quantum dots. Biomaterials. 2012;33:1238–44. https://doi.org/10.1016/j.biomaterials.2011.10.070.
Priyadarshini E, Rawat K. Au@ carbon dot nanoconjugates as a dual mode enzyme-free sensing platform for cholesterol. J Mater Chem B. 2017;5(27):5425–32. https://doi.org/10.1039/C7TB01345K.
Kang EB, Sharker SM, In I, Park SY. Plunoric mimicking florescent carbon nanoparticles conjugated with doxorubicin via acid-cleavable linkage for tumor-targeted drug delivery and bioimaging. J Ind Eng Chem. 2016;43:150–7. https://doi.org/10.1016/j.jiec.2016.08.001.
Chen FH, Zhang LM, Chen QT, Zhang Y, Zhang ZJ. Synthesis of a novel magnetic drug delivery system composed of doxorubicin-conjugated Fe3O4 nanoparticle cores and a PEG-functionalized porous silica shell. Chem Commun. 2010;46:8633–5. https://doi.org/10.1039/C0CC02577A.
Chen FH, Gao Q, Ni JZ. The grafting and release behaviour of doxorubicin from Fe3O4@ SiO2 core–shell structure nanoparticles via an acid cleaving amide bond: the potential for magnetic targeting drug delivery. Nanotechnology. 2008;19:165103. https://doi.org/10.1088/0957-4484/19/16/165103.
Du JZ, Du XJ, Mao CQ, Wang J. Tailor-made dual pH-sensitive polymer doxorubicin nanoparticles for efficient anticancer drug delivery. J Am Chem Soc. 2011;133:17560–3. https://doi.org/10.1021/ja207150n.
Akbarzadeh A, Samiei M, Joo SW, Anzaby M, Hanifehpour Y, Nasrabadi HT, et al. Synthesis, characterization and in vitro studies of doxorubicin-loaded magnetic nanoparticles grafted to smart copolymers on A549 lung cancer cell line. J Nanobiotechnol. 2012;10:46. https://doi.org/10.1186/1477-3155-10-46.
KievitFM WFY, Fang C, Mok H, Wang K, Silber JR, Ellenbogen RG, et al. Doxorubicin loaded iron oxide nanoparticles overcome multidrugresistance in cancer in vitro. J Control Release: Off J Control Release Soc. 2011;152:76–83. https://doi.org/10.1016/j.jconrel.2011.01.024.
Dreis S, Rothweiler F, Michaelis M, Cinat J Jr, Kreuter J, Langer K. Preparation, characterisation and maintenance of drug efficacy ofdoxorubicin-loaded human serum albumin (HSA) nanoparticles. Int J Pharm. 2007;341:207–14. https://doi.org/10.1016/j.ijpharm.2007.03.036.
Gautier J, Munnier E, Paillard A, et al. A pharmaceutical study of doxorubicin-loaded PEGylated nanoparticles for magnetic drug targeting. Int J Pharm. 2012;423:16–25. https://doi.org/10.1016/j.ijpharm.2011.06.010.
SemkinaA, Abakumov M, Grinenkoc N, Abakumovd A, Skorikovb A, Mironovae E, Davydova G, Majouga AG, Nukolov N, Kabanov A, Chekhonin V. Core–shell–corona doxorubicin-loaded superparamagnetic Fe3O4nanoparticles for cancer theranostics. Colloids and Surfaces B: Biointerfaces. 2015; 136, 1073–1080. https://doi.org/10.1016/j.colsurfb.2015.11.009.
Kim D, Jeong YY, Jon SA. Drug-loaded aptamer−gold nanoparticle bioconjugate for combined CT imaging and therapy of prostate cancer. ACS Nano. 2010;4:3689–96. https://doi.org/10.1021/nn901877h.
Zhou S, Starkov A, Froberg MK, Leino RL, Wallace KB. Cumulative and irreversible cardiac mitochondrial dysfunction induced by doxorubicin. Cancer Res. 2001;61:771–7.
Mizutani H, Tada-Oikawa S, Hiraku Y, Kojima M, Kawanishi S. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 2005;76:1439–53. https://doi.org/10.1016/j.lfs.2004.05.040.
Acknowledgements
EP is thankful to Department of Science and Technology-Science and Engineering Board, New Delhi, India, for fellowship under National Postdoctoral Scheme (PDF/2017/000024). Authors are grateful to Advanced Instrumentation Research Facility, Jawaharlal Nehru University, New Delhi, for confocal imaging studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Priyadarshini, E., Rajamani, P. Acid-Liable Cleavage of Doxorubicin@Plunoric-Carbon Dots in Multiplexed Bioimaging and Drug Delivery. AAPS PharmSciTech 21, 322 (2020). https://doi.org/10.1208/s12249-020-01871-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01871-1