Abstract
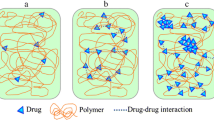
Polymer additives have been widely reported to affect the crystallization of amorphous drugs, while the underlying mechanism is poorly understood. The present study aims to investigate the relationship between the crystal growth and the molecular mobility of amorphous nifedipine (NIF) in the presence and absence of low-concentration poly(ethylene oxide) (PEO). The addition of 3% w/w PEO yields approximately a 5-fold increase in the crystal growth rate of NIF in the glassy matrix and a 10-fold increase in the supercooled liquid. Broadband dielectric spectroscopy is performed to investigate the molecular mobility of amorphous pure NIF system and NIF doped with low-concentration PEO. With 3% w/w PEO, the structural relaxation time τα of amorphous NIF significantly decreases, indicating an increase in the global molecular mobility. However, the increase of the molecular mobility is insufficient to explain the 5- to 10-fold increase of the crystal growth rate at the same τα scale. Moreover, we compare the accelerating effect of PEO in NIF-PEO systems to other PEO-doped systems. The accelerating effect of low-concentration PEO on the crystal growth of amorphous drugs is found to be independent of the Flory–Huggins interaction, Tg of the drug, or the increase of the global molecular mobility. These findings suggest that an in-depth understanding regarding the effects of polymer additives on the crystallization of drugs should consider the localized mobility of the host molecules near the crystal-liquid interface.






Similar content being viewed by others
References
Van den Mooter G. The use of amorphous solid dispersions: a formulation strategy to overcome poor solubility and dissolution rate. Drug Discov Today Technol. 2012;9(2):79–85.
Xu W, Xie H, Cao Q, Shi L, Cao Y, Zhu X, et al. Enhanced dissolution and oral bioavailability of valsartan solid dispersions prepared by a freeze-drying technique using hydrophilic polymers. Drug Deliv. 2016;23(1):41–8.
Yan H, Chris H. Amorphous solid dispersions: utilization and challenges in drug discovery and development. J Pharm Sci. 2015;104(10):3237–58.
Schittny A, Huwyler J, Puchkov M. Mechanisms of increased bioavailability through amorphous solid dispersions: a review. Drug Deliv. 2020;27(1):110–27.
Powell CT, Cai T, Hasebe M, Gunn EM, Gao P, Zhang G, et al. Low-concentration polymers inhibit and accelerate crystal growth in organic glasses in correlation with segmental mobility. J Phys Chem B. 2013;117(35):10334–41.
Ishida H, Wu T, Yu L. Sudden rise of crystal growth rate of nifedipine near T(g) without and with polyvinylpyrrolidone. J Pharm Sci. 2007;96(5):1131–8.
Kestur US, Lee H, Santiago D, Rinaldi C, Won Y, Taylor LS. Effects of the molecular weight and concentration of polymer additives, and temperature on the melt crystallization kinetics of a small drug molecule. Cryst Growth Des. 2010;10(8):3585–95.
Eerdenbrugh BV, Taylor LS. An ab initio polymer selection methodology to prevent crystallization in amorphous solid dispersions by application of crystal engineering principles. CrystEngComm. 2011;13(20):6171–8.
Kestur US, Taylor LS. Role of polymer chemistry in influencing crystal growth rates from amorphous felodipine. Cryst Eng Comm. 2010;12(8):2390–7.
Trasi NS, Taylor LS. Effect of additives on crystal growth and nucleation of amorphous flutamide. Cryst Growth Des. 2012;12(6):3221–30.
Trasi NS, Taylor LS. Effect of polymers on nucleation and crystal growth of amorphous acetaminophen. Cryst Eng Comm. 2012;14(16):5188–97.
Sato T, Taylor LS. Acceleration of the crystal growth rate of low molecular weight organic compounds in supercooled liquids in the presence of polyhydroxybutyrate. CrystEngComm. 2017;19:80–7.
Kestur US, Taylor LS. Evaluation of the crystal growth rate of felodipine polymorphs in the presence and absence of additives as a function of temperature. Cryst Growth Des. 2013;13(10):4349–54.
Zhang S, Britten JF, Chow AHL, Lee TWY. Impact of crystal structure and polymer excipients on the melt crystallization kinetics of itraconazole polymorphs. Cryst Growth Des. 2017;17(6):3433–42.
Tian B, Gao W, Tao X, Tang X, Taylor LS. Impact of polymers on the melt crystal growth rate of indomethacin polymorphs. Cryst Growth Des. 2017;17(12):6467–76.
Cai T, Zhu L, Yu L. Crystallization of organic glasses: effects of polymer additives on bulk and surface crystal growth in amorphous nifedipine. Pharm Res. 2011;28(10):2458–66.
Huang C, Powell CT, Sun Y, Cai T, Yu L. Effect of low-concentration polymers on crystal growth in molecular glasses: a controlling role for polymer segmental mobility relative to host dynamics. J Phys Chem B. 2017;121(8):1963–71.
Fung MH, DeVault M, Kuwata KT, Suryanarayanan R. Drug-excipient interactions: effect on molecular mobility and physical stability of ketoconazole-organic acid coamorphous systems. Mol Pharm. 2018;15(3):1052–61.
Mistry P, Mohapatra S, Gopinath T, Vogt FG, Suryanarayanan R. Role of the strength of drug-polymer interactions on the molecular mobility and crystallization inhibition in ketoconazole solid dispersions. Mol Pharm. 2015;12(9):3339–50.
Zhang J, Shi Q, Tao J, Peng Y, Cai T. Impact of polymer enrichment at the crystal-liquid Interface on crystallization kinetics of amorphous solid dispersions. Mol Pharm. 2019;16(3):1385–96.
Zhang J, Shi Q, Guo M, Liu Z, Cai T. Melt crystallization of indomethacin polymorphs in the presence of poly(ethylene oxide): selective enrichment of the polymer at the crystal-liquid Interface. Mol Pharm. 2020;17(6):2064–71.
Kothari K, Ragoonanan V, Suryanarayanan R. Influence of molecular mobility on the physical stability of amorphous pharmaceuticals in the supercooled and glassy states. Mol Pharm. 2014;11(9):3048–55.
Kothari K, Ragoonanan V, Suryanarayanan R. The role of polymer concentration on the molecular mobility and physical stability of nifedipine solid dispersions. Mol Pharm. 2015;12(5):1477–84.
Kothari K, Ragoonanan V, Suryanarayanan R. The role of drug-polymer hydrogen bonding interactions on the molecular mobility and physical stability of nifedipine solid dispersions. Mol Pharm. 2015;12(1):162–70.
Mehta M, Ragoonanan V, McKenna GB, Suryanarayanan R. Correlation between molecular mobility and physical stability in pharmaceutical glasses. Mol Pharm. 2016;13(4):1267–77.
Mehta M, Suryanarayanan R. Accelerated physical stability testing of amorphous dispersions. Mol Pharm. 2016;13(8):2661–6.
Grzybowska K, Capaccioli S, Paluch M. Recent developments in the experimental investigations of relaxations in pharmaceuticals by dielectric techniques at ambient and elevated pressure. Adv Drug Deliv Rev. 2016;100:158–82.
Mistry P, Suryanarayanan R. Strength of drug–polymer interactions: implications for crystallization in dispersions. Cryst Growth Des. 2016;16(9):5141–9.
Mehta M, Kothari K, Ragoonanan V, Suryanarayanan R. Effect of water on molecular mobility and physical stability of amorphous pharmaceuticals. Mol Pharm. 2016;13(4):1339–46.
Fung MH, Suryanarayanan R. Use of a plasticizer for physical stability prediction of amorphous solid dispersions. Cryst Growth Des. 2017;17(8):4315–25.
Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed. 2010;49(36):6288–308.
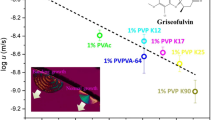
Shi Q, Zhang C, Su Y, Zhang J, Zhou D, Cai T. Acceleration of crystal growth of amorphous griseofulvin by low-concentration poly(ethylene oxide): aspects of crystallization kinetics and molecular mobility. Mol Pharm. 2017;14(7):2262–72.
Shi Q, Zhang J, Zhang C, Jiang J, Tao J, Zhou D, et al. Selective acceleration of crystal growth of indomethacin polymorphs by low-concentration poly(ethylene oxide). Mol Pharm. 2017;14(12):4694–704.
Marsac PJ, Shamblin SL, Taylor LS. Theoretical and practical approaches for prediction of drug–polymer miscibility and solubility. Pharm Res. 2006;23:2417–26.
Havriliak S, Negami S. A complex plane representation of dielectric and mechanical relaxation processes in some polymers. Polymer. 1967;8:161–210.
Madejczyk O, Kaminska E, Tarnacka M, Dulski M, Jurkiewicz K, Kaminski K, et al. Studying the crystallization of various polymorphic forms of nifedipine from binary mixtures with the use of different experimental techniques. Mol Pharm. 2017;14(6):2116–25.
Madejczyk O, Minecka A, Kamińska E, Heczko D, Tarnacka M, Wolnica K, et al. Studying the crystal growth of selected active pharmaceutical ingredients from single- and two-component systems above and below the glass transition temperature. Cryst Growth Des. 2019;19(2):1031–40.
Gunn E, Guzei IA, Cai T, Yu L. Polymorphism of nifedipine: crystal structure and reversible transition of the metastable β polymorph. Cryst Growth Des. 2012;12(4):2037–43.
Zhu L, Wong L, Yu L. Surface-enhanced crystallization of amorphous nifedipine. Mol Pharm. 2008;5(6):921–6.
Sun Y, Xi H, Ediger MD, Yu L. Diffusionless crystal growth from glass has precursor in equilibrium liquid. J Phys Chem B. 2008;112(3):661–4.
Shi Q, Cai T. Fast crystal growth of amorphous griseofulvin: relations between bulk and surface growth modes. Cryst Growth Des. 2016;16(6):3279–86.
Musumeci D, Powell CT, Ediger MD, Yu L. Termination of solid-state crystal growth in molecular glasses by fluidity. J Phys Chem Lett. 2014;5(10):1705–10.
Wang K, Sun CC. Crystal growth of celecoxib from amorphous state: polymorphism, growth mechanism, and kinetics. Cryst Growth Des. 2019;19(6):3592–600.
Vogel H. Das Temperaturabhangigkeitgesetz der Viskosität von Flüssigkeiten. J Phys Z. 1921;22:645–6.
Fulcher GS. Analysis of recent measurements of the viscosity of glasses. J Am Ceram Soc. 1925;8:339–55.
Tammann G, Hesse W. Die Abhängigkeit der Viscosität von der Temperatur bie unterkühlten Flüssigkeiten. Z Anorg Allg Chem. 1926;156:245–57.
Sun Y, Xi H, Ediger MD, Richert R, Yu L. Diffusion-controlled and “Diffusionless” crystal growth near the glass transition temperature: relation between liquid dynamics and growth kinetics of seven ROY polymorphs. J Chem Phys. 2009;131(7):074506.
Acknowledgments
The authors are grateful for financial support of this work by the National Natural Science Foundation of China (Nos.81803452, 21803004) and the Natural Science Foundation of Jiangsu Province (No.BK20180273).
Funding
This study received financial support from the National Natural Science Foundation of China (Nos. 81803452, 21803004) and the Natural Science Foundation of Jiangsu Province (No. BK20180273).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 108 kb)
Rights and permissions
About this article
Cite this article
Shi, Q., Cheng, J., Li, F. et al. Molecular Mobility and Crystal Growth in Amorphous Binary Drug Delivery Systems: Effects of Low-Concentration Poly(Ethylene Oxide). AAPS PharmSciTech 21, 317 (2020). https://doi.org/10.1208/s12249-020-01869-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01869-9