Abstract
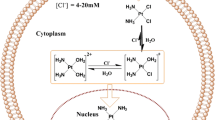
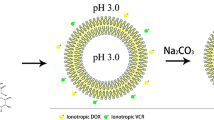
Conventional combination chemotherapy often leads to unsatisfactory clinical outcomes due to the different distribution characteristics in vivo and the superimposed systemic toxicity of the drug cocktail. Co-encapsulated nano preparations have been gradually developed in recent years. In this work, cytarabine (Ara-C)/daunorubicin (DNR) liposomes were prepared by the pH gradient (ADL-pH) and Cu2+ gradient (ADL-Cu) methods. Ara-C did not show significant release from either ADL-Cu or ADL-pH in vitro during 168 h, which related to its logPoct. Different drug-loading patterns showed different release characteristics of DNR due to the different existence forms, ADL-pH contains the citrate form, while in ADL-Cu, there is the Cu2+ complex. To evaluate the release behavior, daunorubicin liposome (DL) and daunorubicin-Cu2+ complex (DNR-Cu) were prepared. The addition of EDTA in the release medium significantly increased the release rate of DNR from DL-Cu, while lower pH accelerated DNR release from both DL-pH and DL-Cu. The PK confirmed that ADL-Cu and ADL-pH could prolong the drug circulation time, and ADL-Cu had a mean retention time 1.5 times that of ADL-pH. Furthermore, both liposomes allowed the two drugs to maintain a relatively constant plasma concentration ratio for a prolonged time. Cytotoxicity assays showed that Ara-C/DNR with a molar ratio of 5:1 and 3:1 exhibited an excellent synergistic effect, which was more obvious at 5:1. In vitro antitumor results revealed that ADL-Cu exhibited more cytotoxicity than ADL-pH. All factors tested in this work suggest the considerable potential of ADL-Cu and ADL-pH for anticancer treatment.











Similar content being viewed by others
Abbreviations
- Ara-C:
-
Cytarabine
- DNR:
-
Daunorubicin
- ADL-pH:
-
Cytarabine/daunorubicin liposome prepared by pH gradient method
- ADL-Cu:
-
Cytarabine/daunorubicin liposome prepared by Cu2+ gradient method
- DL:
-
Daunorubicin liposome
- DNR-Cu:
-
Daunorubicin-Cu2+ complex
- AML:
-
Acute myelogenous leukemia
- DL-passive:
-
Daunorubicin liposome prepared by passive drug-loading method
- DL-Cu:
-
Daunorubicin liposome prepared by Cu2+ gradient method
- DL-pH:
-
Daunorubicin liposome prepared by pH gradient method
- TEM:
-
Transmission electron microscopy
- PK:
-
Pharmacokinetics
- DMSO:
-
Dimethyl sulfoxide
- CI:
-
Combination Index
- MRT:
-
Mean residence time
- ROS:
-
Reactive oxygen species
References
Kim W, Haws H, Mangelson R, Peterson P, Whatcott C, Siddiqui-Jain A, et al. Alvocidib synergizes with cytarabine and daunorubicin (7+3) in preclinical models of acute myeloid leukemia. Haematologica. 2017;102:370.
Trusova VM, Deligeorgiev T, Gorbenko G. Liposomal co-encapsulation of two novel europium complexes and doxorubicin: fluorescence study. J Fluoresc. 2017;27(4):1359–63. https://doi.org/10.1007/s10895-017-2070-x.
Lei J, Cong S, Song M, Zhang W, Peng G, Li X, et al. Combination of doxorubicin with harmine-loaded liposomes exerting synergistic antitumor efficacy. Drug Dev Ind Pharm. 2018;44(4):570–81. https://doi.org/10.1080/03639045.2017.1405432.
Maakaron JE, Mims AS. Daunorubicin-cytarabine liposome (CPX-351) in the management of newly diagnosed secondary AML: a new twist on an old cocktail. Best Pract Res Clin Haematol. 2019;32(2):127–33. https://doi.org/10.1016/j.beha.2019.05.005.
Tardi PG, Dos Santos N, Harasym TO, Johnstone SA, Zisman N, Tsang AW, et al. Drug ratio-dependent antitumor activity of irinotecan and cisplatin combinations in vitro and in vivo. Mol Cancer Ther. 2009;8(8):2266–75. https://doi.org/10.1158/1535-7163.MCT-09-0243.
Harasym TO, Liboiron BD, Mayer LD. Drug ratio-dependent antagonism: a new category of multidrug resistance and strategies for its circumvention. Methods Mol Biol. 2010;596:291–323. https://doi.org/10.1007/978-1-60761-416-6_13.
Camacho KM, Menegatti S, Vogus DR, Pusuluri A, Fuchs Z, Jarvis M, et al. DAFODIL: a novel liposome-encapsulated synergistic combination of doxorubicin and 5FU for low dose chemotherapy. J Control Release. 2016;229:154–62. https://doi.org/10.1016/j.jconrel.2016.03.027.
Raut LS. Novel formulation of cytarabine and daunorubicin: a new hope in AML treatment. South Asian J Cancer. 2015;3:38–40. https://doi.org/10.4103/2278-330X.149950.
Jensen IS, Wu E, Sacks NC, Cyr PL, Chung KC. Budget impact analysis of using daunorubicin-cytarabine liposome in patients with newly diagnosed therapy-related AML or AML and myelodysplasia-related changes. Am Health Drug Benefits. 2018;11(No.7):380–6.
Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. Cpx-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(No.26):2684–92. https://doi.org/10.1200/JCO.2017.77.6112.
Krauss AC, Gao X, Li L, Manning ML, Patel P, Fu W, et al. FDA approval summary: (daunorubicin and cytarabine) liposome for injection for the treatment of adults with high-risk acute myeloid leukemia. Clin Cancer Res. 2019;25(9):2685–90. https://doi.org/10.1158/1078-0432.CCR-18-2990.
Tardi P, Johnstone S, Harasym N, Xie S, Harasym T, Zisman N, et al. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33(1):129–39. https://doi.org/10.1016/j.leukres.2008.06.028.
Kim M, Williams S. Daunorubicin and cytarabine liposome in newly diagnosed therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes. Ann Pharmacother. 2018;52(8):792–800. https://doi.org/10.1177/1060028018764923.
Tolcher AWML. Improving combination cancer therapy: the CombiPlex((R)) development platform. Future Oncol. 2018;14(No.13):1317–32. https://doi.org/10.1007/s10637-012-9839-1.
Kim HP, Gerhard B, Harasym TO, Mayer LD, Hogge DE. Liposomal encapsulation of a synergistic molar ratio of cytarabine and daunorubicin enhances selective toxicity for acute myeloid leukemia progenitors as compared to analogous normal hematopoietic cells. Exp Hematol. 2011;39(7):741–50. https://doi.org/10.1016/j.exphem.2011.04.001.
Dicko A, Kwak S, Frazier AA, Mayer LD, Liboiron BD. Biophysical characterization of a liposomal formulation of cytarabine and daunorubicin. Int J Pharm. 2010;391(1–2):248–59. https://doi.org/10.1016/j.ijpharm.2010.02.014.
Sha X, Guo J, Chen Y, Fang X. Effect of phospholipid composition on pharmacokinetics and biodistribution of epirubicin liposomes. J Liposome Res. 2012;22(1):80–8. https://doi.org/10.3109/08982104.2011.627513.
Eloy JO, Claro de Souza M, Petrilli R, Barcellos JP, Lee RJ, Marchetti JM. Liposomes as carriers of hydrophilic small molecule drugs: strategies to enhance encapsulation and delivery. Colloids Surf B: Biointerfaces. 2014;123:345–63. https://doi.org/10.1016/j.colsurfb.2014.09.029.
Mayer LD, Tardi P, Louie AC. CPX-351: a nanoscale liposomal co-formulation of daunorubicin and cytarabine with unique biodistribution and tumor cell uptake properties. Int J Nanomedicine. 2019;14:3819–30. https://doi.org/10.2147/IJN.S139450.
Lei M, Ma M, Pang X, Tan F, Li N. A dual pH/thermal responsive nanocarrier for combined chemo-thermotherapy based on a copper-doxorubicin complex and gold nanorods. Nanoscale. 2015;7(38):15999–6011. https://doi.org/10.1039/c5nr04353k.
Azadeh Kheirolomoom LMM, Lai C-Y, Lindfors HA, Seo JW, Paoli EE, Watson KD, et al. Copper-doxorubicin as a nanoparticle cargo retains efficacy with minimal toxicity. Mol Pharm. 2010;7(No.6):1948–58. https://doi.org/10.1021/mp100245u.
Alfayez M, Kantarjian H, Kadia T, Ravandi-Kashani F, Daver N. CPX-351 (vyxeos) in AML. Leuk Lymphoma. 2020;61(2):288–97. https://doi.org/10.1080/10428194.2019.1660970.
Liu J, Chi D, Pan S, Zhao L, Wang X, Wang D, et al. Effective co-encapsulation of doxorubicin and irinotecan for synergistic therapy using liposomes prepared with triethylammonium sucrose octasulfate as drug trapping agent. Int J Pharm. 2019;557:264–72. https://doi.org/10.1016/j.ijpharm.2018.12.072.
Russell LM, Hultz M, Searson PC. Leakage kinetics of the liposomal chemotherapeutic agent Doxil: the role of dissolution, protonation, and passive transport, and implications for mechanism of action. J Control Release. 2018;269:171–6. https://doi.org/10.1016/j.jconrel.2017.11.007.
Beraldo H, Garnier-Suillerot A, Tosi L. Copper(II)-adriamycin complexes. A circular dichroism and resonance Raman study. Inorg Chem. 1983;22(26):4117–24. https://doi.org/10.1021/ic00168a058.
Nakamura K, Yoshino K, Yamashita K, Kasukawa H. Designing a novel in vitro drug-release-testing method for liposomes prepared by pH-gradient method. Int J Pharm. 2012;430(1–2):381–7. https://doi.org/10.1016/j.ijpharm.2012.04.011.
Lee RJ, Wang S, Turk MJ, Low PS. The effects of pH and intraliposomal buffer strength on the rate of liposome content release and intracellular drug delivery. Biosci Rep. 1998;18(2):69–78. https://doi.org/10.1023/A:1020132226113.
Kang JH, Jang WY, Ko YT. The effect of surface charges on the cellular uptake of liposomes investigated by live cell imaging. Pharm Res. 2017;34(4):704–17. https://doi.org/10.1007/s11095-017-2097-3.
Barenholz Y. Liposome application: problems and prospects. Curr Opin Colloid Interface Sci. 2001;6(No.1):66–77. https://doi.org/10.1016/S1359-0294(00)00090-X.
Fenske PRCMJHMBBTDMLDMDB. Influence of pH gradients on the transbilayer transport of drugs, lipids, peptides and metal ions into large unilamellar vesicles. Biochim Biophys Acta. 1997;1331(No.2):187–211. https://doi.org/10.1016/S0304-4157(97)00006-3.
Gagdhattdlas T. Long-circulating liposomes for drug delivery in cancer therapy: a review of biodistribution studies in tumor-bearing animals. Adv Drug Deliv Rev. 1997;24(No.2):337–44. https://doi.org/10.1016/S0169-409X(96)00476-0.
GASHB Y. Pharmacokinetics of pegylated liposomal doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42(No.5):419–36.
Acknowledgments
Thanks to the help of my tutor Xing Tang and my sister Lingli Zhou, and Amanda Pearce is gratefully thanked for correcting English grammar of the manuscript.
Funding
This work was supported by the National Mega-project for Innovative Drugs (No. 2019ZX09721001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were approved by the Ethics Committee of Shenyang Pharmaceutical University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All animal experiments are in line with guidelines for the care and use of laboratory animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, J., Zhou, L., Zhang, Y. et al. Contrastive Studies of Cytarabine/Daunorubicin Dual-Loaded Liposomes Prepared by pH Gradient and Cu2+ Gradient Method. AAPS PharmSciTech 21, 325 (2020). https://doi.org/10.1208/s12249-020-01867-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01867-x