Abstract
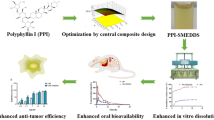
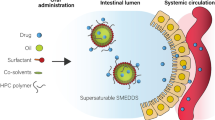
10-Hydroxycamptothecin (HCPT) is a DNA inhibitor of topoisomerase I and exerts antitumor activities against various types of cancer. However, reversible conversion from a pharmacologically active lactone form to an inactive carboxylate form of HCPT and poor water solubility hamper its clinical applications. To overcome these shortcomings, we designed a fine self-microemulsifying drug delivery system (SMEDDS) for HCPT to effectively protect HCPT in its active lactone form as well as improving dissolution rates. A formulation of HCPT-SMEDDS that contained ethyl oleate, D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS), and polyethylene glycol 400 (PEG400) was optimized by using the central composite design and response surface methodology. Following 1:100 aqueous dilution of the optimized HCPT-SMEDDS, the droplet size of resulting microemulsions was 25.6 ± 0.7 nm, and the zeta potential was − 15.2 ± 0.4 mV. The optimized HCPT-SMEDDS appeared to stabilize the lactone moiety of HCPT with 73.6% being present in the pharmacologically active lactone forms in simulated intestinal fluid, but only 45.7% for free HCPT. Furthermore, the physically stable formulation showed the active lactone form predominated in HCPT-SMEDDS (> 95%) for 6 months under the accelerated storage condition. Meanwhile, the optimized SMEDDS formulation also significantly improved dissolution rates and membrane permeability of the lactone form of HCPT. Therefore, HCPT-SMEDDS involved designing for the ease of manufacture, and provided a potent oral dosage form for preserving its active lactone form as well as enhancing the dissolution rate.








Similar content being viewed by others
References
Hsiang YH, Liu LF. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988;48:1722–6.
Garcia-Carbonero R, Supko JG. Current perspectives on the clinical experience, pharmacology, and continued development of the camptothecins. Clin Cancer Res. 2002;8:641–61.
Zhang L, Hu Y, Jiang X, Yang C, Lu W, Yang YH. Camptothecin derivative-loaded poly(caprolactone-co-lactide)-b-PEG-b-poly(caprolactone-co- lactide) nanoparticles and their biodistribution in mice. J Control Release. 2004;96:135–48.
Lv F, Liu D, Cong H, Shen Y, Yu B. Synthesis, self-assembly and drug release behaviors of a bottlebrush polymer-HCPT prodrug for tumor chemotherapy. Colloids Surf B: Biointerfaces. 2019;181:278–84.
Liu Y, Li D, Guo X, Xu H, Li Z, Zhang Y, et al. A pH-responsive prodrug delivery system of 10-HCPT for controlled release and tumor targeting. Int J Nanomedicine. 2017;12:2227–42.
Zhou T, Tang X, Zhang W, Feng J, Wu W. Preparation and in vitro and in vivo evaluations of 10-hydroxycamptothecin liposomes modified with stearyl glycyrrhetinate. Drug Deliv. 2019;26:673–9.
Zhang L, Yang M, Wang Q, Li Y, Guo R, Jiang X, et al. 10-Hydroxycamptothecin loaded nanoparticles: preparation and antitumor activity in mice. J Control Release. 2007;119:153–62.
Yang L, Hong J, Di J, Guo Y, Han M, Liu M, et al. 10-Hydroxycamptothecin (HCPT) nanosuspensions stabilized by mPEG1000-HCPT conjugate: high stabilizing efficiency and improved antitumor efficacy. Int J Nanomedicine. 2017;12:3681–95.
Fassberg J, Stella VJ. A kinetic and mechanistic study of the hydrolysis of camptothecin and some analogues. J Pharm Sci. 1992;81:676–84.
O'Leary J, Muggia FM. Camptothecins: a review of their development and schedules of administration. Eur J Cancer. 1998;34:1500–8.
Venditto VJ, Simanek EE. Cancer therapies utilizing the camptothecins: a review of the in vivo literature. Mol Pharm. 2010;7:307–49.
Deepa P, Krutika KS. Self micro-emulsifying drug delivery system: formulation development and biopharmaceutical evaluation of lipophilic drugs. Curr Drug Deliv. 2009;6:419–24.
Na YG, Byeon JJ, Wang M, Huh HW, Son GH, Jeon SH, et al. Strategic approach to developing a self-microemulsifying drug delivery system to enhance antiplatelet activity and bioavailability of ticagrelor. Int J Nanomedicine. 2019;14:1193–212.
Craig DQM, Lievens HSR, Pitt KG, Storey DE. An investigation into the physico-chemical properties of self-emulsifying systems using low frequency dielectric spectroscopy, surface tension measurements and particle size analysis. Int J Pharm. 1993;96:147–55.
Li P, Ghosh A, Wagner RF, Krill S, Joshi YM, Serajuddin ATM. Effect of combined use of nonionic surfactant on formation of oil-in-water microemulsions. Int J Pharm. 2005;288:27–34.
Neslihan Gursoy R, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58:173–82.
Zhang Z, Tan S, Feng S-S. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33:4889–906.
Yu L, Bridgers A, Polli J, Vickers A, Long S, Roy A, et al. Vitamin E-TPGS increases absorption flux of an HIV protease inhibitor by enhancing its solubility and permeability. Pharm Res. 1999;16:1812–7.
Neophytou CM, Mesaritis A, Gregoriou G, Constantinou AI. d-a-Tocopheryl polyethylene glycol 1000 succinate and a small-molecule survivin suppressant synergistically induce apoptosis in SKBR3 breast cancer cells. Sci Rep. 2019;9:14375.
Cui J, Yu B, Zhao Y, Zhu W, Li H, Lou H, et al. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int J Pharm. 2009;371:148–55.
Yan B, Wang Y, Ma Y, Zhao J, Liu Y, Wang L. In vitro and in vivo evaluation of poly (acrylic acid) modified mesoporous silica nanoparticles as pH response carrier for β-elemene self-micro emulsifying. Int J Pharm. 2019;572:118768.
Guidance for industry Q1A (R2) stability testing of new drug substances and products. https://www.fda.gov/media/71707/download.
Na YG, Byeon JJ, Wang M, Huh HW, Kim MK, Bang KH, et al. Statistical approach for solidifying ticagrelor loaded self-microemulsifying drug delivery system with enhanced dissolution and oral bioavailability. Mater Sci Eng C Mater Biol Appl. 2019;104:109980.
Gao L, Wang X, Ma J, Hao D, Wei P, Zhou L, et al. Evaluation of TPGS-modified thermo-sensitive Pluronic PF127 hydrogel as a potential carrier to reverse the resistance of P-gp-overexpressing SMMC-7721 cell lines. Colloids Surf B: Biointerfaces. 2016;140:307–16.
Baek MK, Lee JH, Cho YH, Kim HH, Lee GW. Self-microemulsifying drug-delivery system for improved oral bioavailability of pranlukast hemihydrate: preparation and evaluation. Int J Nanomedicine. 2013;8:167–76.
Xie M, Wu J, Ji L, Jiang X, Zhang J, Ge M, et al. Development of triptolide self-microemulsifying drug delivery system and its anti-tumor effect on gastric cancer xenografts. Front Oncol. 2019;9:978.
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, et al. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12:263–71.
Gundogdu E, Karasulu HY, Koksal C, Karasulu E. The novel oral imatinib microemulsions: physical properties, cytotoxicity activities and improved Caco-2 cell permeability. J Microencapsul. 2013;30:132–42.
Silva R, Vilas-Boas V, Carmo H, Dinis-Oliveira RJ, Carvalho F, de Lourdes Bastos M, et al. Modulation of P-glycoprotein efflux pump: induction and activation as a therapeutic strategy. Pharmacol Ther. 2015;149:1–123.
Funding
The authors received financial support from the Science and Technology Foundation of Guangzhou (Project No. 201904010425) and the Research Project of Traditional Chinese Medicine of the Health Bureau of Shenzhen Guangming District (Project No. GM2019020026).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 905 kb)
Rights and permissions
About this article
Cite this article
Li, R., Wang, Y., Yang, Q. et al. Enhanced Stability of the Pharmacologically Active Lactone Form of 10-Hydroxycamptothecin by Self-Microemulsifying Drug Delivery Systems. AAPS PharmSciTech 21, 324 (2020). https://doi.org/10.1208/s12249-020-01860-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01860-4