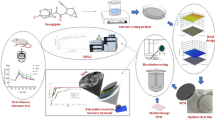
Abstract
The aim of this study was to introduce smectite clay matrices as a drug delivery carrier for the development of amorphous solid dispersions (ASD). Indomethacin (IND) was processed with two different smectite clays, magnesium aluminium and lithium magnesium sodium silicates, using hot melt extrusion (HME) to prepare solid dispersions. Scanning electron microscopy (SEM), powdered X-ray diffraction (PXRD), and differential scanning calorimetry (DSC) were used to examine the physical form of the drug. Energy-dispersive X-ray (EDX) spectroscopy was used to investigate the drug distribution, and attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopic analysis was done to detect any chemical interaction between these two kinds. Both PXRD and DSC analyses showed that drug–clay solid dispersion contained IND in amorphous form. EDX analysis showed a uniform IND dispersion in the extruded powders. ATR-FTIR data presented possible drug and clay interactions via hydrogen bonding. In vitro drug dissolution studies revealed a lag time of about 2 h in the acidic media and a rapid release of IND at pH 7.4. The work demonstrates that preparation of amorphous solid dispersion using inorganic smectite clay particles can effectively increase the dissolution rate of IND.








Similar content being viewed by others
References
Keserü GM, Makara GM. The influence of lead discovery strategies on the properties of drug candidates. Nat Rev Drug Discov. 2009;8(3):203–12.
Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44(1):235–49.
Mithu MSH, Ross SA, Alexander BD, Douroumis D. Solid state thermomechanical engineering of high-quality pharmaceutical salts via solvent free continuous processing. Green Chem. 2020;22(2):540–9.
Ross SA, Ward A, Basford P, Mcallister M, Douroumis D. Coprocessing of pharmaceutical cocrystals for high quality and enhanced physicochemical stability. 2018;
Malamatari M, Taylor KMG, Malamataris S, Douroumis D, Kachrimanis K. Pharmaceutical nanocrystals: production by wet milling and applications. Drug Discov Today. 2018;23(3):534–47.
Mithu S, Malamatari M, Douroumis D. Crystal engineering of ketoconazole solid forms with dicarboxylic acids using mechanochemistry. J Pharm Pharmacol. 2017;69:9–10.
Upadhye SB, Kulkarni SJ, Majumdar S, Avery MA, Gul W, ElSohly MA, et al. Preparation and characterization of inclusion complexes of a hemisuccinate ester prodrug of Δ 9-tetrahydrocannabinol with modified beta-cyclodextrins. AAPS PharmSciTech. 2010;11(2):509–17.
Wang L, Li H, Wang S, Liu R, Wu Z, Wang C, et al. Enhancing the antitumor activity of berberine hydrochloride by solid lipid nanoparticle encapsulation. AAPS PharmSciTech. 2014;15(4):834–44.
Alqahtani F, Belton P, Ward A, Asare-Addo K, Qi S. An investigation into the use of low quantities of functional additives to control drug release from hot melt extruded solid dispersions for poorly soluble drug delivery. Int J Pharm. 2020;119172.
Alhijjaj M, Belton P, Fabian L, Wellner N, Reading M, Qi S. Novel thermal imaging method for rapid screening of drug–polymer miscibility for solid dispersion based formulation development. Mol Pharm. 2018;15(12):5625–36.
Alhijjaj M, Yassin S, Reading M, Zeitler JA, Belton P, Qi S. Characterization of heterogeneity and spatial distribution of phases in complex solid dispersions by thermal analysis by structural characterization and X-ray micro computed tomography. Pharm Res. 2017;34(5):971–89.
Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersion systems. J Pharm Sci. 1971;60(9):1281–302.
Abend S, Lagaly G. Sol–gel transitions of sodium montmorillonite dispersions. Appl Clay Sci. 2000;16(3–4):201–27.
Carretero MI, Pozo M. Clay and non-clay minerals in the pharmaceutical industry: part I. Excipients and medical applications. Appl Clay Sci. 2009;46:73–80.
Viseras C, Lopez-Galindo A. Pharmaceutical applications of some Spanish clays (sepiolite, palygorskite, bentonite): some preformulation studies. Appl Clay Sci. 1999;14(1–3):69–82.
Adebisi AO, Conway BR, Asare-Addo K. The influence of fillers on theophylline release from clay matrices. Am J Pharmacol Sci. 2015;3(5):120–5.
Gonçalves MLCM, Lyra MAM, Oliveira FJVE, Rolim LA, Nadvorny D, Vilarinho ACSG, et al. Use of phyllosilicate clay mineral to increase solubility olanzapine. J Therm Anal Calorim. 2017;127(2):1743–50.
Kim MH, Choi G, Elzatahry A, Vinu A, Choy Y Bin, Choy JH. Review of clay-drug hybrid materials for biomedical applications: administration routes. Clay Clay Miner 2016;64(2):115–130.
McPhee C, Reed J, Zubizarreta I. Core sample preparation. In:Developments in Petroleum Science: Elsevier; 2015. p. 135–79.
Jung H, Kim H, Bin Y, Hwang S, Choy J. Laponite-based nanohybrid for enhanced solubility and controlled release of itraconazole. Int J Pharm. 2008;349:283–90.
Bahl D, Hudak J, Bogner RH. Comparison of the ability of various pharmaceutical silicates to amorphize and enhance dissolution of indomethacin upon co-grinding. Pharm Dev Technol. 2008;13(3):255–69.
Löbenberg R, Amidon GL. Modern bioavailability, bioequivalence and biopharmaceutics classification system. new scientific approaches to international regulatory standards. Eur J Pharm Biopharm. 2000;50(1):3–12.
Alsaidan SM, Alsughayer AA, Eshra AG. Improved dissolution rate of indomethacin by adsorbents. Drug Dev Ind Pharm. 1998 Jan 1;24(4):389–94.
Zhang W, Zhang C. ning, He Y, Duan B yan, Yang G yi, Ma W dong, et al. Factors affecting the dissolution of indomethacin solid dispersions. AAPS PharmSciTech. 2017;18(8):3258–73.
Angadi G, Narayana Rao Narasimha Murthy H, Ramakrishna S, Firdosh S, Nagappa R, Munishamaiah K. Effect of screw configuration on the dispersion of nanofillers in thermoset polymers. J Polym Eng 2017;37(8):815–825.
Breitenbach J. Melt extrusion: from process to drug delivery technology. Eur J Pharm Biopharm. 2002;54:107–17.
Repka MA, Battu SK, Upadhye SB, Thumma S, Crowley MM, Zhang F, et al. Pharmaceutical applications of hot-melt extrusion: part II. Drug Dev Ind Pharm. 2007;33:1043–57.
Liu H, Zhu L, Wang P, Zhang X, Gogos CG. Effects of screw configuration on indomethacin dissolution behavior in Eudragit E PO. Adv Polym Technol. 2012;31(4):331–42.
Hwang I, Kang C-Y, Park J-B. Advances in hot-melt extrusion technology toward pharmaceutical objectives. J Pharm Investig. 2017;47(2):123–32.
Prasad D, Chauhan H, Atef E. Amorphous stabilization and dissolution enhancement of amorphous ternary solid dispersions: combination of polymers showing drug–polymer interaction for synergistic effects. J Pharm Sci. 2014;103(11):3511–23.
Maniruzzaman M, Nair A, Scoutaris N, Bradley MSA, Snowden MJ, Douroumis D. One-step continuous extrusion process for the manufacturing of solid dispersions. Int J Pharm. 2015;496(1):42–51.
Villar MV, Gómez-Espina R, Gutiérrez-Nebot L. Basal spacings of smectite in compacted bentonite. Appl Clay Sci. 2012;65:95–105.
El-Badry M, Fetih G, Fathy M. Improvement of solubility and dissolution rate of indomethacin by solid dispersions in Gelucire 50/13 and PEG4000. Saudi Pharm J. 2009;17(3):217–25.
Grim RE, Bradley WF. Investigation of the effect of heat on the clay minerals illite and montmorillonite. J Am Ceram Soc. 1940;23(8):242–8.
Corcione CE, Maffezzoli A. Thermochimica acta glass transition in thermosetting clay-nanocomposite polyurethanes. 2009;485:43–8.
Qazvini NT, Chehrazi E. Glass transition behavior and dynamic fragility of PMMA-SAN miscible blend-clay nanocomposites. J Macromol Sci Part B Phys. 2011;50(11):2165–77.
Tabak A, Yilmaz N, Eren E, Caglar B, Afsin B, Sarihan A. Structural analysis of naproxen-intercalated bentonite (Unye). Chem Eng J. 2011;174(1):281–8.
Kevadiya BD, Patel HA, Joshi GV, Abdi SHR, Bajaj HC. Montmorillonite-alginate composites as a drug delivery system : intercalation and in vitro release of diclofenac sodium. Indi. 2010;72(6):732–7.
Ghadiri M, Chrzanowski W, Lee WH, Fathi A, Dehghani F, Rohanizadeh R. Physico-chemical, mechanical and cytotoxicity characterizations of Laponite®/alginate nanocomposite. Appl Clay Sci. 2013;85:64–73.
Patel HA, Somani RS, Bajaj HC, Jasra RV. Preparation and characterization of phosphonium montmorillonite with enhanced thermal stability. Appl Clay Sci. 2007;35(3–4):194–200.
A RM, Kebriaee A, Keshavarz M, Ahmadi A, Mohtat B. Preparation and in-vitro evaluation of indomethacin nanoparticles. 2010;18(3):185–92.
Fini A, Cavallari C, Ospitali F. Raman and thermal analysis of indomethacin/PVP solid dispersion enteric microparticles. Eur J Pharm Biopharm. 2008;70(1):409–20.
Kocbek P, Baumgartner S, Kristl J. Preparation and evaluation of nanosuspensions for enhancing the dissolution of poorly soluble drugs. Int J Pharm. 2006;312(1–2):179–86.
Jatav S, Joshi, M Y. Chemical stability of Laponite in aqueous media. y Sci 2014;97–98(August):72–77.
Trivedi V, Nandi U, Maniruzzaman M, Coleman NJ. Intercalated theophylline-smectite hybrid for pH-mediated delivery. Drug Deliv Transl Res. 2018;8:1781–9.
Netpradit S, Thiravetyan P, Towprayoon S. Adsorption of three azo reactive dyes by metal hydroxide sludge: effect of temperature, pH, and electrolytes. J Colloid Interface Sci. 2004;270(2):255–61.
Tabak A, Eren E, Afsin B, Caglar B. Determination of adsorptive properties of a Turkish sepiolite for removal of reactive blue 15 anionic dye from aqueous solutions. J Hazard Mater. 2009;161(2–3):1087–94.
Tabak A, Baltas N, Afsin B, Emirik M, Caglar B, Eren E. Adsorption of reactive red 120 from aqueous solutions by cetylpyridinium-bentonite. J Chem Technol Biotechnol. 2010;85(9):1199–207.
Tombacz E, Szekeres M. Colloidal behavior of aqueous montmorillonite suspensions: the specific role of pH in the presence of indifferent electrolytes. Appl Clay Sci. 2004;27(1–2):75–94.
Alonzo DE, Zhang GGZ, Zhou D, Gao Y, Taylor LS. Understanding the behavior of amorphous pharmaceutical systems during dissolution. Pharm Res. 2010;27(4):608–18.
Funding
This project has received funding from the Interreg 2 Seas programme 2014–2020 co-funded by the European Regional Development Fund under subsidy contract 2S01-059_IMODE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Guest Editors: Feng Zhang, Michael Repka and Suresh Bandari
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nandi, U., Mithu, M.S., Hurt, A.P. et al. Drug–Smectite Clay Amorphous Solid Dispersions Processed by Hot Melt Extrusion. AAPS PharmSciTech 21, 276 (2020). https://doi.org/10.1208/s12249-020-01813-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01813-x