Abstract
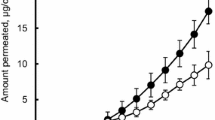
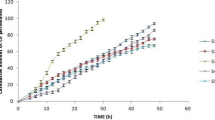
The aim of this study was to develop a suitable drug-in-adhesive patch for transdermal delivery of koumine. Acrylic polymer Duro-Tak® 87-4287, which contains hydroxyl groups, may significantly enhance the skin permeation of koumine from transdermal patches containing 0.93–3.72% koumine. Among permeation enhancers, 10% azone showed the greatest potential and increased the flux of koumine to 1.48-fold that of the control. Therefore, an optimized patch formulation containing 3.72% koumine and 10% azone in Duro-Tak® 87-4287 that offers good physical properties was selected for an in vivo pharmacokinetic study using rats. The maximal plasma drug concentration (Cmax) of koumine after transdermal administration (4 mg/patch) was 25.80 ± 1.51 ng/mL, which was in the range of those after oral administration (3 mg/kg and 15 mg/kg). The time to the maximal concentration (Tmax) and the half-life (t1/2) of the drug with transdermal administration were 3.96 ± 0.46 h and 21.10 ± 1.36 h, respectively, which were longer than those with oral administration. Furthermore, the area under the concentration-time curve (AUC0–72 h) of 898.20 ± 45.57 ng·h/mL for the transdermal patch was much higher than that for oral administration (15 mg/kg). In conclusion, the drug-in-adhesive patch containing koumine provides a steady plasma koumine level and sustained release in vivo and can be an effective means of transdermal delivery for koumine.






Similar content being viewed by others
References
Dutt V, Thakur S, Dhar VJ, Sharma A. The genus Gelsemium: an update. Pharmacogn Rev. 2010;4:185–94.
Rujjanawate C, Kanjanapothi D, Panthong A. Pharmacological effect and toxicity of alkaloids from Gelsemium elegans Benth. J Ethnopharmacol. 2003;89:91–5.
Su YP, Shen J, Xu Y, Zheng M, Yu CX. Preparative separation of alkaloids from Gelsemium elegans Benth. using pH-zone-refining counter-current chromatography. J Chromatogr A. 2011;1218:3695–8.
Fang L, Zhou J, Lin YL, Wang X, Sun QL, Li JL, et al. Large-scale separation of alkaloids from Gelsemium elegans by pH-zone-refining counter-current chromatography with a new solvent system screening method. J Chromatogr A. 2013;1307:80–5.
Zhang XH, Chen Y, Gao B, Luo DL, Wen YY, Ma XL. Apoptotic effect of koumine on human breast cancer cells and the mechanism involved. Cell Biochem Biophys. 2015;72:411–6.
Zhang LL, Huang CQ, Zhang ZY, Wang ZR, Lin JM. Therapeutic effects of koumine on psoriasis: an experimental study in mice. J First Mil Univ. 2005;25:547–9.
Qiu HQ, Xu Y, Jin GL, Yang J, Liu M, Li SP, et al. Koumine enhances spinal cord 3α-hydroxysteroid oxidoreductase expression and activity in a rat model of neuropathic pain. Mol Pain. 2015;11:46.
Xu Y, Qiu HQ, Liu H, Liu M, Huang ZY, Yang J, et al. Effects of koumine, an alkaloid of Gelsemium elegans Benth., on inflammatory and neuropathic pain models and possible mechanism with allopregnanolone. Pharmacol Biochem Behav. 2012;101:504–14.
Liu M, Huang HH, Yang J, Su YP, Lin HW, Lin LQ, et al. The active alkaloids of Gelsemium elegans Benth. are potent anxiolytics. Psychopharmacology. 2013;225:839–51.
Chen CJ, Zhong ZF, Xin ZM, Hong LH, Su YP, Yu CX. Koumine exhibits anxiolytic properties without inducing adverse neurological effects on functional observation battery, open-field and Vogel conflict tests in rodents. J Nat Med. 2017;71:397–408.
Yang J, Cai HD, Zeng YL, Chen ZH, Fang MH, Su YP, et al. Effects of koumine on adjuvant- and collagen-induced arthritis in rats. J Nat Prod. 2016;79:2635–43.
Sharif K, Sharif A, Jumah F, Oskouian R, Tubbs RS. Rheumatoid arthritis in review: clinical, anatomical, cellular and molecular points of view. Clin Anat. 2018;31:216–23.
Yu CX, Xu Y, Yang J, Su YP, Cai HD. Chinese Patent No. ZL 201110174657.6 Beijing: National Intellectual Property Administration, PRC. 2012.
Yu CX, Xu Y, Yang J, Su YP, Cai HD. U.S. Patent No. 9,078,890 B2 Washington, DC: U.S. Patent and Trademark Office. 2015.
Wang L, Sun Q, Zhao N, Wen YQ, Song Y, Meng FH. Ultra-liquid chromatography tandem mass spectrometry (UPLC-MS/MS)-based pharmacokinetics and tissue distribution study of koumine and the detoxification mechanism of Glycyrrhiza uralensis Fisch on Gelsemium elegans Benth. Molecules. 2018;23:1693–706.
Wang L, Wen YQ, Meng FH. Simultaneous determination of gelsemine and koumine in rat plasma by UPLC-MS/MS and application to pharmacokinetic study after oral administration of Gelsemium elegans Benth extract. Biomed Chromatogr. 2018;32:e4201–20.
Al Hanbali OA, Khan HMS, Sarfraz M, Arafat M, Ijaz S, Hameed A. Transdermal patches: design and current approaches to painless drug delivery. Acta Pharma. 2019;69:197–215.
Subedi RK, Oh SY, Chun MK, Choi HK. Recent advances in transdermal drug delivery. Arch Pharm Res. 2010;33:339–51.
Liu CT, Wang QW, Wang CH. The structure of koumine. Acta ChimicaSinica. 1986;4:73–92.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26.
Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin's barrier function. Pharm Sci Technolo Today. 2000;3:318–26.
Tan HS, Pfister WR. Pressure-sensitive adhesives for transdermal drug delivery systems. Pharm Sci Technolo Today. 1999;2:60–9.
Lobo S, Sachdeva S, Goswami T. Role of pressure-sensitive adhesives in transdermal drug delivery systems. Ther Deliv. 2016;7:33–48.
Venkatraman S, Gale R. Skin adhesives and skin adhesion: 1. Transdermal drug delivery systems. Biomaterials. 1998;19:1119–36.
Rhee YS, Nguyen T, Park ES, Chi SC. Formulation and biopharmaceutical evaluation of a transdermal patch containing aceclofenac. Arch Pharm Res. 2013;36:602–7.
Tuntiyasawasdikul S, Limpongsa E, Jaipakdee N, Sripanidkulchai B. A monolithic drug-in-adhesive patch of methoxyflavones from Kaempferiaparviflora: in vitro and in vivo evaluation. Int J Pharm. 2015;478:486–95.
Marwah H, Garg T, Goyal AK, Rath G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016;23:564–78.
Shahid N, Siddique MI, Razzaq Z, Katas H, Waqas MK, Rahman KU. Fabrication and characterization of matrix type transdermal patches loaded with tizanidine hydrochloride: potential sustained release delivery system. Drug Dev Ind Pharm. 2018;44:2061–70.
Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, vol. IV. China Medical Science Press, Beijing. 2015.
Sinn Aw M, Kurian M, Losic D. Non-eroding drug-releasing implants with ordered nanoporous and nanotubular structures: concepts for controlling drug release. Biomater Sci. 2014;2:10–34.
Chen JZ, Li Y, Xiao JP, Wu SS, Song HW. Development of a sensitive and rapid UPLC-MS/MS method for the determination of koumine in rat plasma: application to a pharmacokinetic study. Biomed Chromatogr. 2013;27:736–40.
Ye LX, Su YP, Cao DX, Lin J, Yu CX. Content determination and stability of koumine by UPLC. Chin J Mod Appl Pharm. 2015;32:471–4.
Shaw A, Coleone-Carvalho AC, Hollingshurst J, Draper M, Machado Neto JG. Development of a new test cell to measure cumulative permeation of water-insoluble pesticides with low vapor pressure through protective clothing and glove materials. Ind Health. 2017;55:555–63.
Ashara KC, Shah KV. Association of permeability and lipid content of membrane. Folia Med (Plovdiv). 2017;59:203–9.
Nair AB, Gupta S, Al-Dhubiab BE, Jacob S, Shinu P, Shah J, et al. Effective therapeutic delivery and bioavailability enhancement of pioglitazone using drug in adhesive transdermal patch. Pharmaceutics. 2019;11:359–73.
Taghizadeh SM, Soroushnia A, Mohamadnia F. Preparation and in vitro evaluation of a new fentanyl patch based on functional and non-functional pressure sensitive adhesives. AAPS PharmSciTech. 2010;11:278–84.
Liu C, Quan P, Li SS, Zhao YS, Fang L. A systemic evaluation of drug in acrylic pressure sensitive adhesive patch in vitro and in vivo: the roles of intermolecular interaction and adhesive mobility variation in drug controlled release. J Control Release. 2017;252:83–94.
Peng CC, Chauhan A. Ion transport in silicone hydrogel contact lenses. J Membrane Sci. 2012;399-400:95–105.
Stoughton RR, McClure WO. Azone®: a new non-toxic enhancer of cu’taneous penetration. Drug Dev Ind Pharm. 1983;9:725–44.
Chang L, Fan DD, Duan ZG, Jia LP, Zhu CH, Ma XX. The transdermal absorption study of human-like collagen. Adv Mater Res. 1781-1785;2012:415–7.
Haq A, Michniak-Kohn B. Effects of solvents and penetration enhancers on transdermal delivery of thymoquinone: permeability and skin deposition study. Drug Deliv. 2018;25:1943–9.
Chen Y, Quan P, Liu XC, Wang ML, Fang L. Novel chemical permeation enhancers for transdermal drug delivery. Asian J Pharm Sci. 2014;9:51–64.
Liu C, Fang L. Drug in adhesive patch of zolmitriptan: formulation and in vitro/in vivo correlation. AAPS PharmSciTech. 2015;16:1245–53.
da Silva ER, de Freitas ZM, GitiranaLde B, Ricci-Júnior E. Improving the topical delivery of zinc phthalocyanine using oleic acid as a penetration enhancer: in vitro permeation and retention. Drug Dev Ind Pharm. 2011;37:569–75.
Tuntiyasawasdikul S, Limpongsa E, Jaipakdee N, Sripanidkulchai B. Transdermal permeation of Kaempferia parviflora methoxyflavones from isopropyl myristate-based vehicles. AAPS PharmSciTech. 2014;15:947–55.
Jung E, Lee EY, Choi HK, Ban SJ, Choi SH, Kim JS, et al. Development of drug-in-adhesive patch formulations for transdermal delivery of fluoxetine: in vitro and in vivo evaluations. Int J Pharm. 2015;487:49–55.
Barry BW. Mode of action of penetration enhancers in human skin. J Control Release. 1987;6:85–97.
Atef E, Altuwaijri N. Using raman spectroscopy in studying the effect of propylene glycol, oleic acid, and their combination on the rat skin. AAPS PharmSciTech. 2018;19:114–22.
Khan MZU, Makreski P, Murtaza G. Preparation, optimization, in vitro evaluation and ex vivo permeation studies of rinasteride loaded gel formulations prepared by using response surface methodology. Curr Drug Deliv. 2018;15:1312–22.
Diez-Sales O, Watkinson AC, M. Herraez-Dominguez M, Javaloyes C, Hadgraft J. A mechanistic investigation of the in vitro human skin permeation enhancing effect of Azone®. Int J Pharm. 1996;129:33–40.
Kim JH, Choi HK. Effect of additives on the crystallization and the permeation of ketoprofen from adhesive matrix. Int J Pharm. 2002;236:81–5.
Jain P, Banga AK. Inhibition of crystallization in drug-in-adhesive-type transdermal patches. Int J Pharm. 2010;394:68–74.
Jung E, Kang YP, Yoon IS, Kim JS, Kwon SW, Chung SJ, et al. Effect of permeation enhancers on transdermal delivery of fluoxetine: in vitro and in vivo evaluation. Int J Pharm. 2013;456:362–9.
Bajpai AK, Shukla SK, Bhanu S, Kankane S. Responsive polymers in controlled drug delivery. Prog Polym Sci. 2008;33:1088–118.
de Velde F, de Winter BC, Koch BC, van Gelder T, Mouton JW. COMBACTE-NET consortium. Non-linear absorption pharmacokinetics of amoxicillin: consequences for dosing regimens and clinical breakpoints. J Antimicrob Chemother. 2016;71:2909–17.
Yáñez JA, Remsberg CM, Sayre CL, Forrest ML, Davies NM. Flip-flop pharmacokinetics--delivering a reversal of disposition: challenges and opportunities during drug development. Ther Deliv. 2011;2:643–72.
Zeng Q, Xie L, Zhang J, Vuong C, Potter B, Aylor S, et al. Improving relative bioavailability of oral imidazolidinedione by reducing particle size using homogenization and ultra-sonication. Mil Med. 2019;184:106–13.
Funding
The research was supported by the Industry-University-Research Cooperation Project of Fujian Province (grant number 2017Y4007), the Drug Innovation Major Project of China (grant number 2018ZX09711001-003-024), the Central Financial Support Universities Funds of China (grant number 2018L3008), and the Joint Funds for the Innovation of Science and Technology of Fujian Province (grant number 2018Y9074).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Su, Y., Lu, W., Fu, X. et al. Formulation and Pharmacokinetic Evaluation of a Drug-in-Adhesive Patch for Transdermal Delivery of Koumine. AAPS PharmSciTech 21, 297 (2020). https://doi.org/10.1208/s12249-020-01793-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01793-y