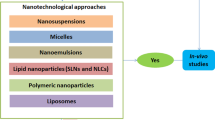
Abstract
Approximately 40% of compounds in clinical drug development suffer from solubility and bioavailability challenges. Evidence from literature demonstrates the growing interest to utilize flavonoids as potential compounds owing to their widespread therapeutic utility in various ailments. Nobiletin (NOB), one such dietary polymethoxylated flavonoid found in citrus fruits, has multiple pharmacological effects such as antioxidant, anti-microbial, anti-cancer, and anti-inflammatory. It is useful in cancer, inflammatory bowel diseases, atherosclerosis, obesity, and Alzheimer’s disease. Although preclinical studies demonstrate the therapeutic utility of NOB, it suffers from serious biopharmaceutical limitations such as low aqueous solubility (below 1 μg/ml), poor permeability across biological barriers, and low bioavailability. To overcome these biopharmaceutical challenges associated with NOB, the use of advanced formulations and nanotechnology-based strategies appears to be a promising approach to potentiate its therapeutic action. Multiple reviews cover the various therapeutic benefits of NOB in various diseases; however, there is an absence of a comprehensive review that focuses on the formulation development strategies of NOB. The purpose of this review is to provide a concise perspective on NOB as a candidate molecule for formulation development. The manuscript covers various aspects related to NOB, such as its chemistry, physicochemical properties, and pharmacological effects. This is also a thorough review of various formulation development strategies with advances made in the past years to improve the solubility, bioavailability, and therapeutic efficacy of NOB. The review also contains information related to toxicity and patents involving NOB and its formulation.




Similar content being viewed by others
References
Hattori T, Tagawa H, Inai M, Kan T, Kimura S, Itai S, et al. Transdermal delivery of nobiletin using ionic liquids. Sci Rep. 2019;9(1):1–11.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23(1–3):3–25.
Julio A, Lima SAC, Reis S, de Almeida TS, Fonte P. Development of ionic liquid-polymer nanoparticle hybrid systems for delivery of poorly soluble drugs. J Drug Deliv Sci Technol. 2019;100915.
Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20.
Ghadi R, Dand N. BCS class IV drugs: highly notorious candidates for formulation development. J Control Release. 2017;248:71–95.
Dressman J, Butler J, Hempenstall J, Reppas C. The BCS: where do we go from here? Pharm Technol. 2001;25(7):68–77.
Di AC, Angelico R. Advanced nanotechnologies for enhancing the bioavailability of silymarin: a state of the art. 2019.
Kumar S, Kesharwani SS, Mathur H, Tyagi M, Bhat GJ, Tummala H. Molecular complexation of curcumin with pH sensitive cationic copolymer enhances the aqueous solubility, stability and bioavailability of curcumin. Eur J Pharm Sci. 2016;82:86–96.
Kesharwani SS, Ahmad R, Bakkari MA, Rajput MK, Dachineni R, Valiveti CK, et al. Site-directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBD): formulation development, characterization and pharmacological evaluation. J Control Release. 2018;290:165–79.
Tang C, Hoo PC-X, Tan LT-H, Pusparajah P, Khan TM, Lee L-H, et al. Golden needle mushroom: a culinary medicine with evidenced-based biological activities and health promoting properties. Front Pharmacol. 2016;7:474.
Alam MN, Almoyad M, Huq F. Polyphenols in colorectal cancer: current state of knowledge including clinical trials and molecular mechanism of action. Biomed Res Int. 2018;2018:1–29.
Sankaranarayanan R, Valiveti CK, Kumar DR, Kesharwani SS, Seefeldt T, Scaria J, et al. The flavonoid metabolite 2, 4, 6-trihydroxybenzoic acid is a CDK inhibitor and an anti-proliferative agent: a potential role in cancer prevention. Cancers. 2019;11(3):427.
Dachineni R, Kumar DR, Callegari E, Kesharwani SS, Sankaranarayanan R, Seefeldt T, et al. Salicylic acid metabolites and derivatives inhibit CDK activity: novel insights into aspirin's chemopreventive effects against colorectal cancer. Int J Oncol. 2017;51(6):1661–73.
Thilakarathna SH, Rupasinghe H. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. 2013;5(9):3367–87.
Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1):230S–42S.
Li J, Yang Y, Ning E, Peng Y, Zhang J. Mechanisms of poor oral bioavailability of flavonoid Morin in rats: from physicochemical to biopharmaceutical evaluations. Eur J Pharm Sci. 2019;128:290–8.
Di Costanzo A, Angelico R. Formulation strategies for enhancing the bioavailability of silymarin: the state of the art. Molecules. 2019;24(11):2155.
Goh JXH, Tan LT-H, Goh JK, Chan KG, Pusparajah P, Lee LH, et al. Nobiletin and derivatives: functional compounds from citrus fruit peel for colon cancer chemoprevention. Cancers. 2019;11(6):867.
Huang H, Li L, Shi W, Liu H, Yang J, Yuan X, et al. The multifunctional effects of nobiletin and its metabolites in vivo and in vitro. Evid Based Complement Alternat Med. 2016;2016:1–14.
Moon JY, Cho M, Ahn KS, Cho SK. Nobiletin induces apoptosis and potentiates the effects of the anticancer drug 5-fluorouracil in p53-mutated SNU-16 human gastric cancer cells. Nutr Cancer. 2013;65(2):286–95.
Chen C, Ono M, Takeshima M, Nakano S. Antiproliferative and apoptosis-inducing activity of nobiletin against three subtypes of human breast cancer cell lines. Anticancer Res. 2014;34(4):1785–92.
Abe S, Hirose S, Nishitani M, Yoshida I, Tsukayama M, Tsuji A, et al. Citrus peel polymethoxyflavones, sudachitin and nobiletin, induce distinct cellular responses in human keratinocyte HaCaT cells. Biosci Biotechnol Biochem. 2018;82(12):2064–71.
Borah N, Gunawardana S, Torres H, McDonnell S, Van Slambrouck S. 5,6,7,3′,4′,5'-Hexamethoxyflavone inhibits growth of triple-negative breast cancer cells via suppression of MAPK and Akt signaling pathways and arresting cell cycle. Int J Oncol. 2017;51(6):1685–93.
Chiou Y-S, Zheng Y-N, Tsai M-L, Lai C-S, Ho C-T, Pan M-H. 5-Demethylnobiletin more potently inhibits colon cancer cell growth than nobiletin in vitro and in vivo. J Food Bioact. 2018;2:91–7.
Du Q, Chen H. The methoxyflavones in Citrus reticulata Blanco cv. ponkan and their antiproliferative activity against cancer cells. Food Chem. 2010;119(2):567–72.
Kunimasa K, Ikekita M, Sato M, Ohta T, Yamori Y, Ikeda M, et al. Nobiletin, a citrus polymethoxyflavonoid, suppresses multiple angiogenesis-related endothelial cell functions and angiogenesis in vivo. Cancer Sci. 2010;101(11):2462–9.
Miyamoto S, Yasui Y, Tanaka T, Ohigashi H, Murakami A. Suppressive effects of nobiletin on hyperleptinemia and colitis-related colon carcinogenesis in male ICR mice. Carcinogenesis. 2008;29(5):1057–63.
Sousa DP, Pojo M, Pinto AT, Leite V, Serra AT, Cavaco BM. Nobiletin alone or in combination with cisplatin decreases the viability of anaplastic thyroid cancer cell lines. Nutr Cancer. 2020;72(2):352–63.
Tung Y-C, Chou Y-C, Hung W-L, Cheng A-C, Yu R-C, Ho C-T, et al. Polymethoxyflavones: chemistry and molecular mechanisms for cancer prevention and treatment. Curr Pharmacol Rep. 2019;5(2):98–113.
Wang Y, Xie J, Ai Z, Su J. Nobiletin-loaded micelles reduce ovariectomy-induced bone loss by suppressing osteoclastogenesis. Int J Nanomedicine. 2019;14:7839–49.
Yao X, Zhu X, Pan S, Fang Y, Jiang F, Phillips GO, et al. Antimicrobial activity of nobiletin and tangeretin against pseudomonas. Food Chem. 2012;132(4):1883–90.
Li S, Sang S, Pan M-H, Lai C-S, Lo C-Y, Yang CS, et al. Anti-inflammatory property of the urinary metabolites of nobiletin in mouse. Bioorg Med Chem Lett. 2007;17(18):5177–81.
Liao W, Liu Z, Zhang T, Sun S, Ye J, Li Z, et al. Enhancement of anti-inflammatory properties of nobiletin in macrophages by a nano-emulsion preparation. J Agric Food Chem. 2018;66(1):91–8.
Lin N, Sato T, Takayama Y, Mimaki Y, Sashida Y, Yano M, et al. Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem Pharmacol. 2003;65(12):2065–71.
Manthey JA, Bendele P. Anti-inflammatory activity of an orange peel polymethoxylated flavone, 3′, 4′, 3, 5, 6, 7, 8-heptamethoxyflavone, in the rat carrageenan/paw edema and mouse lipopolysaccharide-challenge assays. J Agric Food Chem. 2008;56(20):9399–403.
Wu X, Mei Z, Zheng D, Liu Z, Zhu X, Zhou Y, et al. Application of nobiletin in preparation or screening of diabetic cardiomyopathy. Drug. 2018.
Nakajima A, Aoyama Y, Shin E-J, Nam Y, Kim H-C, Nagai T, et al. Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Aβ levels in a triple transgenic mouse model of Alzheimer’s disease (3XTg-AD). Behav Brain Res. 2015;289:69–77.
Seki T, Kamiya T, Furukawa K, Azumi M, Ishizuka S, Takayama S, et al. Nobiletin-rich Citrus reticulata peels, a kampo medicine for Alzheimer’s disease: a case series. Geriatr Gerontol Int. 2013;13(1):236–8.
Hu Y, Shu Q, Liu F, Lei L, Li B, Cao Y, et al. Ca2+−induced whey protein emulgels for the encapsulation of crystalline nobiletin: effect of nobiletin crystals on the viscoelasticity. Food Hydrocoll. 2019;94:57–62.
Tsukayama M, Ichikawa R, Yamamoto K, Sasaki T, Kawamura Y. Microwave-assisted rapid extraction of polymethoxyflavones from dried peels of Citrus yuko Hort. ex Tanaka. Nippon Shokuhin Kagaku Kogaku Kaishi J Jpn Soc Food Sci Technol. 2009;56(6):359–62.
Chen S, Cai D, Pearce K, Sun PY, Roberts AC, Glanzman DL. Reinstatement of long-term memory following erasure of its behavioral and synaptic expression in Aplysia. Elife. 2014;3:e03896.
Zheng J, Bi J, Johnson D, Sun Y, Song M, Qiu P, et al. Analysis of 10 metabolites of polymethoxyflavones with high sensitivity by electrochemical detection in high-performance liquid chromatography. J Agric Food Chem. 2015;63(2):509–16.
Zheng J, Song M, Dong P, Qiu P, Guo S, Zhong Z, et al. Identification of novel bioactive metabolites of 5-demethylnobiletin in mice. Mol Nutr Food Res. 2013;57(11):1999–2007.
Koga N, Ohta C, Kato Y, Haraguchi K, Endo T, Ogawa K, et al. In vitro metabolism of nobiletin, a polymethoxy-flavonoid, by human liver microsomes and cytochrome P450. Xenobiotica. 2011;41(11):927–33.
Li S, Wang Z, Sang S, Huang MT, Ho CT. Identification of nobiletin metabolites in mouse urine. Mol Nutr Food Res. 2006;50(3):291–9.
Wang M. Biotransformation of polymethoxyflavones and its implication on biological activities. 2017.
Xu L, He Y, Guo X, Lu Y, Wang C, Wang Z. Identification of metabolites of nobiletin in rats using ultra-performance liquid chromatography coupled with triple-quadrupole mass spectrometry. Yao xue xue bao Acta Pharm Sin. 2011;46(12):1483–7.
Wu X, Song M, Wang M, Zheng J, Gao Z, Xu F, et al. Chemopreventive effects of nobiletin and its colonic metabolites on colon carcinogenesis. Mol Nutr Food Res. 2015;59(12):2383–94.
Xiong Y, Chen D, Yu C, Lv B, Peng J, Wang J, et al. Citrus nobiletin ameliorates experimental colitis by reducing inflammation and restoring impaired intestinal barrier function. Mol Nutr Food Res. 2015;59(5):829–42.
Nagase H, Omae N, Omori A, Nakagawasai O, Tadano T, Yokosuka A, et al. Nobiletin and its related flavonoids with CRE-dependent transcription-stimulating and neuritegenic activities. Biochem Biophys Res Commun. 2005;337(4):1330–6.
Matsuzaki K, Yamakuni T, Hashimoto M, Haque AM, Shido O, Mimaki Y, et al. Nobiletin restoring β-amyloid-impaired CREB phosphorylation rescues memory deterioration in Alzheimer’s disease model rats. Neurosci Lett. 2006;400(3):230–4.
Gao Z, Gao W, Zeng S-L, Li P, Liu E-H. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J Funct Foods. 2018;40:498–509.
Lin SC, Chen MC, Li S, Lin CC, Wang TT. Antiviral activity of nobiletin against chikungunya virus in vitro. Antivir Ther. 2017;22(8):689–97.
Rooprai HK, Kandanearatchi A, Maidment S, Christidou M, Trillo-Pazos G, Dexter DT, et al. Evaluation of the effects of swainsonine, captopril, tangeretin and nobiletin on the biological behaviour of brain tumour cells in vitro. Neuropathol Appl Neurobiol. 2001;27(1):29–39.
Kim H, Moon JY, Mosaddik A, Cho SK. Induction of apoptosis in human cervical carcinoma HeLa cells by polymethoxylated flavone-rich Citrus grandis Osbeck (Dangyuja) leaf extract. Food Chem Toxicol. 2010;48(8–9):2435–42.
Li S, Pan M-H, Lo C-Y, Tan D, Wang Y, Shahidi F, et al. Chemistry and health effects of polymethoxyflavones and hydroxylated polymethoxyflavones. J Funct Foods. 2009;1(1):2–12.
Luque-Alcaraz AG, Lizardi J, Goycoolea FM, Valdez MA, Acosta AL, Iloki-Assanga SB, et al. Characterization and antiproliferative activity of nobiletin-loaded chitosan nanoparticles. J Nanomater. 2012;2012:1–7.
Huang Y, Wu D, Bao M, Li B, Liang H. Coordination driven self-assembly for enhancing the biological stability of nobiletin. J Mol Liq. 2019;292:111420.
Yao J, Lu Y, Zhou JP. Preparation of nobiletin in self-microemulsifying systems and its intestinal permeability in rats. J Pharm Pharm Sci. 2008;11(3):22–9.
Sun G, Lei L, Chen H, Li B, Cao Y, Li Y. Tailoring of structured hydroxypropyl methylcellulose-stabilized emulsions for encapsulation of nobiletin: modification of the oil and aqueous phases. Food Funct. 2018;9(7):3657–64.
Yao J, Zhou JP, Ping QN, Lu Y, Chen L. Distribution of nobiletin chitosan-based microemulsions in brain following iv injection in mice. Int J Pharm. 2008;352(1–2):256–62.
Onoue S, Uchida A, Takahashi H, Seto Y, Kawabata Y, Ogawa K, et al. Development of high-energy amorphous solid dispersion of nanosized nobiletin, a citrus polymethoxylated flavone, with improved oral bioavailability. J Pharm Sci. 2011;100(9):3793–801.
Huang W, Dou H, Wu H, Sun Z, Wang H, Huang L. Preparation and characterisation of nobiletin-loaded nanostructured lipid carriers. J Nanomater. 2017;2017:1–10.
Jeong HJ, Nam SJ, Song JY, Park SN. Synthesis and physicochemical properties of pH-sensitive hydrogel based on carboxymethyl chitosan/2-hydroxyethyl acrylate for transdermal delivery of nobiletin. J Drug Deliv Sci Technol. 2019;51:194–203.
Wu D, Liang Y, Pei Y, Li B, Liang H. Plant exine capsules based encapsulation strategy: a high loading and long-term effective delivery system for nobiletin. Food Res Int. 2020;127:108691.
Lei L, Zhang Y, He L, Wu S, Li B, Li Y. Fabrication of nanoemulsion-filled alginate hydrogel to control the digestion behavior of hydrophobic nobiletin. LWT-Food Sci Technol. 2017;82:260–7.
Kumar CS. Biological and pharmaceutical nanomaterials. Biological and Pharmaceutical Nanomaterials, by Challa SSR Kumar (Editor), pp 425 ISBN 3–527–31382-6 Wiley-VCH, 2006 425.
Baghel S, Cathcart H, O'Reilly NJ. Polymeric amorphous solid dispersions: a review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J Pharm Sci. 2016;105(9):2527–44.
He Y, Ho C. Amorphous solid dispersions: utilization and challenges in drug discovery and development. J Pharm Sci. 2015;104(10):3237–58.
Iwashita M, Hashizume K, Umehara M, Ishigami T, Onishi S, Yamamoto M, et al. Development of nobiletin–methyl hesperidin amorphous solid dispersion: novel application of methyl hesperidin as an excipient for hot-melt extrusion. Int J Pharm. 2019;558:215–24.
Iwashita M, Umehara M, Onishi S, Yamamoto M, Yamagami K, Ishigami T. Method for producing nobiletin-containing solid dispersion2019 20 June 2019.
Wani RJ, Sharma P, Zhong HA, Chauhan H. Preparation and characterization of griseofulvin solid dispersions. ASSAY Drug Dev Technol. 2020;18(3):109–18.
Hu T, Jiang J-G. Application of nanotechnology in traditional Chinese medicine. Curr Nanosci. 2012;8(3):474–84.
Meng F, Trivino A, Prasad D, Chauhan H. Investigation and correlation of drug polymer miscibility and molecular interactions by various approaches for the preparation of amorphous solid dispersions. Eur J Pharm Sci. 2015;71:12–24.
Jordan S, Murty M, Pilon K. Products containing bitter orange or synephrine: suspected cardiovascular adverse reactions. Can Med Assoc J. 2004;171(8):993–4.
Delaney B, Phillips K, Vasquez C, Wilson A, Cox D, Wang HB, et al. Genetic toxicity of a standardized mixture of citrus polymethoxylated flavones. Food Chem Toxicol. 2002;40(5):617–24.
Qu Y, Liu Y, Chen L, Zhu Y, Xiao X, Wang D, et al. Nobiletin prevents cadmium-induced neuronal apoptosis by inhibiting reactive oxygen species and modulating JNK/ERK1/2 and Akt/mTOR networks in rats. Neurol Res. 2018;40(3):211–20.
Yang G, Li S, Long T, Yang Y, Li Y, inventors. Application of polymethoxyflavone in preparation of prevention drug for cardiovascular inflammation 2017 24 October 2017.
Wu X, Zheng D, Qin Y, Lui Z, Zhu X, inventors. Application of nobiletin in medicine for preventing or treating heart failure.2017 7 July 2017.
Morimoto T, Hasegawa K, Murakami A, Fukuda H, Takahashi K, inventors. Cardiac disease treatment agents containing nobiletin.2011 24 February 2011.
Guthrie N, inventor.Compositions comprising at least one polymethoxyflavone, flavonoid, liminoid, and/or tocotrienol useful in combination therapies for treating diabetes.2014 24 December 2014.
Caramelli G, inventor. Product with blood lipid-loweing activity.2008 2 August 2008.
Zhang T, Liao M, Gong S, Xie X, Sun W, Wang L, et al., inventorsApplication of total flavonoid extract from Citrus aurantium in manufacturing medicines for treating asthma. 2013 20 February 2013.
Acknowledgments
Siddharth S. Kesharwani and Surajit Dey would like to acknowledge the College of Pharmacy, Roseman University of Health Sciences, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Guest Editors: Harsh Chauhan, Abhijit Date and Sonali Dhindwal
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kesharwani, S.S., Mallya, P., Kumar, V.A. et al. Nobiletin as a Molecule for Formulation Development: An Overview of Advanced Formulation and Nanotechnology-Based Strategies of Nobiletin. AAPS PharmSciTech 21, 226 (2020). https://doi.org/10.1208/s12249-020-01767-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01767-0