Abstract
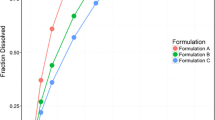
The objective of this study was to develop a dissolution test in order to establish an in vitro–in vivo correlation (IVIVC) model for desvenlafaxine succinate monohydrate (DVSM) extended release (ER) tablets. The in vitro release characteristics of the drug were determined using USP apparatus 1 at 75 rpm, with volume of HCl pH 1.2, acetate buffer solution (ABS) pH 4.5, or phosphate buffer solution (PBS) pH 6.8. In vivo plasma concentrations and pharmacokinetic parameters in healthy volunteers were obtained from a bioequivalence study. The similarity factors f1 and f2 were used to compare the dissolution data. The IVIVC model was developed using fraction dissolved and fraction absorbed of the reference product. For predictability, the results showed that the percentage prediction error (%PE) value of Cmax was 7.63%. The observed low prediction error for Cmax demonstrated that the IVIVC model was valid for this parameter.





Similar content being viewed by others
References
Davanço MG, Campos DR, Carvalho PO. In vitro - in vivo correlation in the development of oral drug formulation: a screenshot of the last two decades. Int J Pharm. 2020;580:1–17. https://doi.org/10.1016/j.ijpharm.2020.119210.
Cardot J, Garrait G, Beyssac E. Use of IVIVC to optimize generic development. Dissolution Technol. 2015;2:44–8. https://doi.org/10.14227/DT220215P44.
Liebowitz MR, Tourian KA. Efficacy, safety, and tolerability of desvenlafaxine 50 mg/d for the treatment of major depressive disorder: a systematic review of clinical trials. Prim Care Companion J Clin Psychiatry. 2010;12:1–19. https://doi.org/10.4088/PCC.09r00845blu.
Deecher DC, Beyer CE, Johnston G, Bray J, Shah S, Abou-Gharbia M, Andree TH. Desvenlafaxine succinate: a new serotonin and norepinephrine reuptake inhibitor 2006;318:657–665. https://doi.org/10.1124/jpet.106.103382.
Nichols AI, Behrle JA, Richards LS, Parker VD, Posener JA, Fruncillo R, et al. The absolute bioavailability of desvenlafaxine in healthy subjects. J Bioequivalence Bioavailab. 2012;4:18–23. https://doi.org/10.4172/jbb.1000105.
Hadfield AF, Shah SM, Winkley MW, Sutherland KW, Provost JA, Aeri P, Shipplett RA, Russel BW, inventors; Wyeth Pharmaceutical Industry Ltd., assignee. Novel succinate salt of o-desmethyl-venlafaxine. International Publication number WO 02/064543 A2. 2002 Aug 22.
PRISTIQ® (desvenlafaxine) Extended-Release Tablets, oral. Prescribing information. Food and drug administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021992s044lbl.pdf. Accessed 14 Dec 2019.
Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS J. 2011;13:519–47. https://doi.org/10.1208/s12248-011-9290-9.
Dave RA, Morris ME. Novel high/low solubility classification methods for new molecular entities. Int J Pharm. 2016;511:111–26. https://doi.org/10.1016/j.ijpharm.2016.06.060.
Franek F, Jarlfors A, Larsen F, Holm P, Steffansen B. In vitro solubility, dissolution and permeability studies combined with semi-mechanistic modeling to investigate the intestinal absorption of desvenlafaxine from an immediate- and extended release formulation. Eur J Pharm Sci. 2015;77:303–13. https://doi.org/10.1016/j.ejps.2015.06.012.
Cardot JM, Beyssac E, Alric M. In vitro–in vivo correlation: importance of dissolution in IVIVC. Dissolution Technol. 2007;14:15–9. https://doi.org/10.14227/DT140107P15.
Bezerra KC, Pinto EC, Cabral LM, de Sousa VP. Development of a dissolution method for gliclazide modified-release tablets using USP apparatus 3 with in vitro–in vivo correlation. Chem Pharm Bull. 2018;66:701–7. https://doi.org/10.1248/cpb.c17-00933.
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry extended release oral dosage forms: development, evaluation, and application of in vitro/in vivo correlations.1997. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/extended-release-oral-dosage-forms-development-evaluation-and-application-vitroin-vivo-correlations. Accessed 14 Dec 2019.
Emami J. In vitro-in vivo correlation: from theory to applications. J Pharm Pharm Sci. 2006;9:31–51.
Jacob S, Nair AB. An updated overview with simple and practical approach for developing in vitro-in vivo correlation. Drug Dev Res. 2018;79:97–110. https://doi.org/10.1002/ddr.21427.
U.S. Food and Drug Administration. Dissolution Methods. Desvenlafaxine succinate. https://www.accessdata.fda.gov/scripts/cder/dissolution/dsp_SearchResults.cfm. Accessed 15 December 2019.
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry Dissolution Testing of Immediate Release Solid Oral Dosage Forms. 1997. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/dissolution-testing-immediate-release-solid-oral-dosage-forms. Accessed 14 Dec 2019.
Predrazzoli-Júnior J, Calafati S, Coelho E, Zanin M, Duarte F, Bonetti F, et al. Avaliação da bioequivalência entre duas formulações de succinato de desvenlafaxina monoidratado – 50 mg comprimido revestido de liberação prolongada – administradas em jejum e pós-prandial em voluntários sadios de ambos os sexos. J Bras Econ da Saúde. 2017;9:198–206. https://doi.org/10.21115/JBES.v9.n2.p198-206.
Wagner BJG, Nelson E. Kinetic analvsis of blood levels and urinary excretion in the absorptive phase after single doses of drug. 1964; 53:1392–1403.
Langenbucher F. Letters to the editor: linearization of dissolution rate curves by the Weibull distribution. J Pharm Pharmacol. 1972;24:979–81.
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, et al. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12:263–71. https://doi.org/10.1208/s12248-010-9185-1.
Klančar U, Markun B, Baumgartner S, Legen I. A novel beads-based dissolution method for the in vitro evaluation of extended release HPMC matrix tablets and the correlation with the in vivo data. AAPS J. 2013;15:267–77. https://doi.org/10.1208/s12248-012-9422-x.
Mirza T, Bykadi SA, Ellison CD, Yang Y, Davit BM, Khan MA. Use of in vitro-in vivo correlation to predict the pharmacokinetics of several products containing a BCS class 1 drug in extended release matrices. Pharm Res. 2013;30:179–90. https://doi.org/10.1007/s11095-012-0861-y.
Mohamed MEF, Trueman S, Othman AA, Han JH, Ju TR, Marroum P. Development of in vitro–in vivo correlation for upadacitinib extended-release tablet formulation. AAPS J The AAPS Journal. 2019;21:1–9. https://doi.org/10.1208/s12248-019-0378-y.
González-García I, Mangas-Sanjuán V, Merino-Sanjuán M, Bermejo M. In vitro-in vivo correlations: general concepts, methodologies and regulatory applications. Drug Dev Ind Pharm. 2015;41:1935–47. https://doi.org/10.3109/03639045.2015.1054833.
European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on quality of oral modified release products.2014. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-oral-modified-release-products_en.pdf. Accessed 16 Dec 2019.
Jambhekar SS, Breen PJ. Drug dissolution: significance of physicochemical properties and physiological conditions. Drug Discov Today. 2013;18:1173–84. https://doi.org/10.1016/j.drudis.2013.08.013.
Vargas M, Villarraga E, Jba V. Bioequivalence study of two 50 mg desvenlafaxine extended release formulations: a randomized, single–dose, open–label, two periods, crossover study. J Bioequivalence Bioavailab. 2014;6:115–8. https://doi.org/10.4172/jbb.1000189.
Li CL, Martini LG, Ford JL, Roberts M. The use of hypromellose in oral drug delivery. J Pharm Pharmacol. 2005;57:533–46. https://doi.org/10.1211/0022357055957.
Cardot J, Davit BM. In vitro – in vivo correlations: tricks and traps. AAPS J. 2012;14:491–9. https://doi.org/10.1208/s12248-012-9359-0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was carried out in a Brazilian Contract Research Organization and had the opinion of the ethics committee approved under No. 572.456
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva, J.D., de Sousa, V.P., Cabral, L.M. et al. In Vitro–In Vivo Correlation for Desvenlafaxine Succinate Monohydrate Extended Release Tablets. AAPS PharmSciTech 21, 195 (2020). https://doi.org/10.1208/s12249-020-01740-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01740-x