Abstract
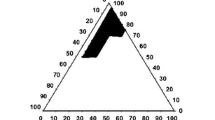
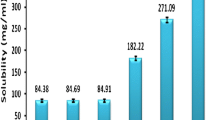
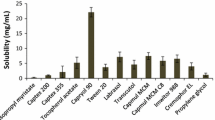
This study aimed to develop a self-nanoemulsifying drug delivery system (SNEDDS) for poorly water-soluble drug stiripentol (STP) with enhanced oral bioavailability. Optimal excipients were selected by constructing pseudo-ternary phase diagrams using determined solubilities of STP, and then the proper composition of SNEDDS was investigated by employing a central composite design method. The optimized SNEDDS was composed of oil (ethyl oleate 39.61%), surfactant (Cremophor® RH 40 43.18%), co-surfactant (1,2-propanediol 17.21%), and STP of 50 mg/mL. The hydrodynamic size, zeta potential, and polydispersity index (PDI) were found to be 45.52 ± 1.99 nm, − 21.67 ± 0.24 mV, and 0.076 ± 0.011, respectively. The optimized STP-SNEDDS showed good stability in accelerated and dilution stability studies. It was also helpful to suppress STP degradation in acidic solution. Compared with STP suspension, STP-SNEDDS presented much faster dissolution rate. STP-SNEDDS successfully resulted in superior levels of Cmax and AUC0 → 6 h (4048.38 ± 704.54 μg/L and 7754.58 ± 1489.37 h μg/L, respectively) to STP suspension (1894.09 ± 1077.64 μg/L and 3556.93 ± 2470.01 h μg/L, respectively). The relative oral bioavailability of STP was 218.01%. The brain biodistribution studies showed that STP-SNEDDS presented significantly higher STP concentrations in the brain at 0.5 h and 1 h than that of STP suspension after administration. These findings indicated that a SNEDDS-based oral formulation of STP would be helpful for increasing its therapeutic potential.








Similar content being viewed by others
References
De Liso P, Chemaly N, Laschet J, et al. Patients with Dravet syndrome in the era of stiripentol: a French cohort cross-sectional study. Epilepsy Res. 2016;125:42–6.
Arends RH, Zhang K, Levy RH, Baillie TA, Shen DD. Stereoselective pharmacokinetics of stiripentol: an explanation for the development of tolerance to anticonvulsant effect. Epilepsy Res. 1994;18(2):91–6.
Strzelczyk A, Kortland LM, Knake S, Rosenow F. Stiripentol for the treatment of super-refractory status epilepticus. Acta Neurol Scand. 2015;132(6):435–9.
Quilichini PP, Chiron C, Ben-Ari Y, Gozlan H. Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABAA-receptor channels. Epilepsia. 2006;47(4):704–16.
Nickels KC, Wirrell EC. Stiripentol in the management of epilepsy. CNS Drugs. 2017;31(5):405–16.
Sada N, Lee S, Katsu T, Otsuki T, Inoue T. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science. 2015;347(6228):1362–7.
Inoue Y, Ohtsuka Y. Long-term safety and efficacy of stiripentol for the treatment of Dravet syndrome: a multicenter, open-label study in Japan. Epilepsy Res. 2015;113:90–7.
Zhang X, Wang H, Zhang T, Zhou X, Wu B. Exploring the potential of self-assembled mixed micelles in enhancing the stability and oral bioavailability of an acid-labile drug. Eur J Pharm Sci. 2014;62:301–8.
Afifi S. Solid dispersion approach improving dissolution rate of stiripentol: a novel antiepileptic drug. Iran J Pharm Res. 2015;14(4):1001–14.
Diacomit 250 mg powder for oral suspension in sachet. Alan Pharmaceuticals. https://www.medicines.org.uk/emc/product/10304/smpc/print. Accessed 31, May 2019.
Lu R, Liu S, Wang Q, Li X. Nanoemulsions as novel oral carriers of stiripentol: insights into the protective effect and absorption enhancement. Int J Nanomedicine. 2015;10:4937–46.
Chatterjee B, Hamed Almurisi S, Ather AMD, et al. Controversies with self-emulsifying drug delivery system from pharmacokinetic point of view. Drug Deliv. 2016;23(9):3639–52.
Karamanidou T, Karidi K, Kammona O, et al. Effective incorporation of insulin in mucus permeating self-nanoemulsifying drug delivery systems. Eur J Pharm Biopharm. 2015;97(Pt A):223–9.
Friedl H, Dünnhaupt S, Hintzen F, Waldner C, Parikh S, Pearson JP, et al. Development and evaluation of a novel mucus diffusion test system approved by self-nanoemulsifying drug delivery systems. J Pharm Sci. 2013;102(12):4406–13.
Date AA, Desai N, Dixit R, Nagarsenker M. Self-nanoemulsifying drug delivery systems: formulation insights, applications and advances. Nanomedicine. 2010;5(10):1595–616.
Qi X, Qin J, Ma N, Chou X, Wu Z. Solid self-microemulsifying dispersible tablets of celastrol: formulation development, characterization and bioavailability evaluation. Int J Pharm. 2014;472(1–2):40–7.
Nan Z, Lijun G, Tao W, Dongqin Q. Evaluation of carbamazepine (CBZ) supersaturatable self- microemulsifying (S-SMEDDS) formulation in-vitro and in-vivo. Iran J Pharm Res. 2012;11(1):257–64.
Knaub K, Sartorius T, Dharsono T, Wacker R, Wilhelm M, Schön C. A novel self-emulsifying drug delivery system (SEDDS) based on VESIsorb® formulation technology improving the oral bioavailability of cannabidiol in healthy subjects. Molecules. 2019;24(16):2967.
Agrawal AG, Kumar A, Gide PS. Self emulsifying drug delivery system for enhanced solubility and dissolution of glipizide. Colloids Surf B Biointerfaces. 2015;126:553–60.
Zhu Y, Xu W, Zhang J, Liao Y, Firempong CK, Adu-Frimpong M, et al. Self-microemulsifying drug delivery system for improved oral delivery of limonene: preparation, characterization, in vitro and in vivo evaluation. AAPS PharmSciTech. 2019;20(4):153–64.
Balakrishnan P, Lee BJ, Oh DH, Kim JO, Lee YI, Kim DD, et al. Enhanced oral bioavailability of coenzyme Q10 by self-emulsifying drug delivery systems. Int J Pharm. 2009;374(1–2):66–72.
Kim DW, Kang JH, Oh DH, Yong CS, Choi HG. Development of novel flurbiprofen-loaded solid self-microemulsifying drug delivery system using gelatin as solid carrier. J Microencapsul. 2012;29(4):323–30.
Zidan AS, Sammour OA, Hammad MA, Megrab NA, Habib MJ, Khan MA. Quality by design: understanding the product variability of a self-nanoemulsified drug delivery system of cyclosporine A. J Pharm Sci. 2007;96(9):2409–23.
Huang Z, Xia J, Li J, et al. Optimization and bioavailability evaluation of self-microemulsifying drug delivery system of the daidzein-nicotinamide complex. RSC Adv. 2016;10:1–10.
Tong Y, Wang Y, Yang M, Yang J, Chen L, Chu X, et al. Systematic development of self-nanoemulsifying liquisolid tablets to improve the dissolution and oral bioavailability of an oily drug, vitamin K1. Pharmaceutics. 2018;10(3):96.
Takahashi R, Imai K, Yamamoto Y, Takahashi Y, Hamano SI, Yoshida H. Determination of stiripentol in plasma by high-performance liquid chromatography with fluorescence detection. Jpn J Pharm Health Care Sci. 2015;41(9):643–50.
Seo YG, Kim DW, Yousaf AM, Park JH, Chang PS, Baek HH, et al. Solid self-nanoemulsifying drug delivery system (SNEDDS) for enhanced oral bioavailability of poorly water-soluble tacrolimus: physicochemical characterisation and pharmacokinetics. J Microencapsul. 2015;32(5):503–10.
Rao SVR, Shao J. Self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of protein drugs: I. formulation development. Int J Pharm. 2008;362(1–2):2–9.
Omari-Siaw E, Zhu Y, Wang H, Peng W, Firempong CK, Wang YW, et al. Hypolipidemic potential of perillaldehyde-loaded self-nanoemulsifying delivery system in high-fat diet induced hyperlipidemic mice: formulation, in vitro and in vivo evaluation. Eur J Pharm Sci. 2016;85:112–22.
U.S. FDA. Inactive Ingredients Database of the FDA https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm.
Cheng G, Hu R, Ye L, Wang B, Gui Y, Gao S, et al. Preparation and in vitro/in vivo evaluation of puerarin solid self-microemulsifying drug delivery system by spherical crystallization technique. AAPS PharmSciTech. 2016;17(6):1336–46.
Cui SX, Nie SF, Li L, Wang CG, Pan WS, Sun JP. Preparation and evaluation of self-microemulsifying drug delivery system containing vinpocetine. Drug Dev Ind Pharm. 2009;35(5):603–11.
Man N, Wang Q, Li H, Adu-Frimpong M, Sun C, Zhang K, et al. Improved oral bioavailability of myricitrin by liquid self-microemulsifying drug delivery systems. J Drug Deliv Sci Tec. 2019;52:597–606.
Bookstaff RC, PaiBir S, Bharaj SS, Kelm GR, Kulick RM, Balm TK, et al. The safety of the use of ethyl oleate in food is supported by metabolism data in rats and clinical safety data in humans. Regul Toxicol Pharmacol. 2003;37(1):133–48.
Bier M, De Graaf J, Zwanikken J, et al. Curvature dependence of the electrolytic liquid-liquid interfacial tension. J Chem Phys. 2009;130(2):024703.
Onoue S, Uchida A, Kuriyama K, Nakamura T, Seto Y, Kato M, et al. Novel solid self-emulsifying drug delivery system of coenzyme Q10 with improved photochemical and pharmacokinetic behaviors. Eur J Pharm Sci. 2012;46(5):492–9.
Nazzal S, Smalyukh II, Lavrentovich OD, Khan MA. Preparation and in vitro characterization of a eutectic based semisolid self-nanoemulsified drug delivery system (SNEDDS) of ubiquinone: mechanism and progress of emulsion formation. Int J Pharm. 2002;235(1–2):247–65.
Spernath A, Yaghmur A, Aserin A, Hoffman RE, Garti N. Food-grade microemulsions based on nonionic emulsifiers: media to enhance lycopene solubilization. J Agric Food Chem. 2002;50(23):6917–22.
Niu B, Yin Z, Qiu N, Yu Y, Huang Q, Zhu Q, et al. Effective management of acute postoperative pain using intravenous emulsions of novel ketorolac prodrugs: in vitro and in vivo evaluations. Eur J Pharm Sci. 2020;149:105344.
Shen Q, Li X, Yuan D, Jia W. Enhanced oral bioavailability of daidzein by self-microemulsifying drug delivery system. Chem Pharm Bull. 2010;58(5):639–43.
Kaasalainen M, Aseyev V, von Haartman E, Karaman DŞ, Mäkilä E, Tenhu H, et al. Size, stability, and porosity of mesoporous nanoparticles characterized with light scattering. Nanoscale Res Lett. 2017;12(1):74.
Moulik SP, Paul BK. Structure, dynamics and transport properties of microemulsions. Adv Colloid Interf Sci. 1998;78(2):99–195.
Hellmig S, Schöning FV, Gadow C, et al. Gastric emptying time of fluids and solids in healthy subjects determined by 13C breath tests: influence of age, sex and body mass index. J Gastroenterol Hepatol. 2006;21(12):1832–8.
Darwish HW, Abdelhameed AS, Attia MI, et al. A stability-indicating HPLC-DAD method for determination of stiripentol: development, validation, kinetics, structure elucidation and application to commercial dosage form. J Anal Methods Chem. 2014;2014:638951.
Qureshi MJ, Mallikarjun C, Kian WG. Enhancement of solubility and therapeutic potential of poorly soluble lovastatin by SMEDDS formulation adsorbed on directly compressed spray dried magnesium aluminometasilicate liquid loadable tablets: a study in diet induced hyperlipidemic rabbits. Asi J Pharm Sci. 2015;10(1):40–56.
Nazzal S, Nutan M, Palamakula A, Shah R, Zaghloul AA, Khan MA. Optimization of a self-nanoemulsified tablet dosage form of ubiquinone using response surface methodology: effect of formulation ingredients. Int J Pharm. 2002;240(1–2):103–14.
Huang J, Gong W, Chen Z, Huang J, Chen Q, Huang H, et al. Emodin self-emulsifying platform ameliorates the expression of FN, ICAM-1 and TGF-β1 in AGEs-induced glomerular mesangial cells by promoting absorption. Eur J Pharm Sci. 2017;99:128–36.
Shen DD, Levy RH, Moor MJ, Savitch JL. Efficacy of stiripentol in the intravenous pentylenetetrazol infusion seizure model in the rat. Epilepsy Res. 1990;7(1):40–8.
Acknowledgments
We acknowledge the valuable help from Dr. Changlai Zhu at Jiangsu Key Laboratory of Nerve Regeneration of Nantong University for the acquisition of the TEM images.
Funding
We thank the financial supports from the China National Natural Science Foundation (81603044), the China Postdoctoral Science Foundation (2017 M611886), the Innovation and Entrepreneurship Training Program for College Students in Jiangsu Province (201710304080Y, 201810304040Z), the Large Instruments Open Foundation of Nantong University (KFJN1852), and the Nantong University Cooperative Innovation Program of Small Molecular Compound R&D-NTU2016–1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Laboratory Animal Ethics Committee of Nantong University. All the animal experiments were performed according to the NIH (National Institutes of Health USA) (2011) Guide for the Care and Use of Laboratory Animals.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dai, Q., Zhang, P., Jin, Y. et al. Using Self-Nanoemulsifying System to Improve Oral Bioavailability of a Pediatric Antiepileptic Agent Stiripentol: Formulation and Pharmacokinetics Studies. AAPS PharmSciTech 21, 192 (2020). https://doi.org/10.1208/s12249-020-01730-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01730-z