Abstract
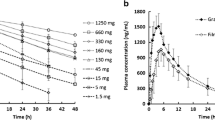
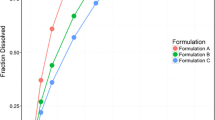
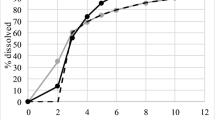
The objective of this study was to develop a novel open-mode two-compartment system dissolution apparatus to simulate the dissolution and absorption of poorly soluble drugs and to establish an in vitro-in vivo correlation (IVIVC). Celecoxib (CEB) was selected as a model drug, and in vitro dissolution was performed using the novel dissolution apparatus with acetate buffers at pH 4.5 containing Tween 80 (0.15%, w/v), at a flow rate of 30 mL/min and an agitation rate of 50 rpm. Cumulative release of all formulations was incomplete at approximately 70–80%, which likely reflected in vivo dissolution. Corresponding pharmacokinetic studies were performed in which twelve healthy male subjects from two bioequivalence studies received either one immediate release (IR) dose of the test (test 1 or test 2) or the reference formulation (Celebrex®, 200 mg). Individual plasma profiles of the formulations were deconvoluted via the Wanger-Nelson method to obtain the mean absorption fractions. A level A correlation was successfully developed with a good R2. The Weibull equation was used to describe the in vitro dissolution and in vivo absorption kinetics. In vitro dissolution correlated with in vivo absorption was applied successfully to predict the in vivo plasma concentrations-time profiles of the CEB formulations. Compared with conventional methods, the novel dissolution device showed great potential for discriminating the dissolution between formulations and generic drugs, which may provide a tool for making in vivo predictions for next bioequivalence trials.







Similar content being viewed by others
Abbreviations
- (IVIVC):
-
In vitro-in vivo correlation
- (CEB):
-
Celecoxib
- (BE):
-
Bioequivalent
- (BCS):
-
Biopharmaceutics Classification System
- (HPLC):
-
High-performance liquid chromatography
- (FDA):
-
Food and Drug Administration
- (SDS):
-
Sodium dodecyl sulfate
References
Emami J. In vitro - in vivo correlation: from theory to applications. J Pharm Pharm Sci. 2006;9(2):169–89.
Deng J, Staufenbiel S, Hao S, Wang B, Dashevskiy A, Bodmeier R. Development of a discriminative biphasic in vitro dissolution test and correlation with in vivo pharmacokinetic studies for differently formulated racecadotril granules. J Control Release. 2017;255:202–9. https://doi.org/10.1016/j.jconrel.2017.04.034.
FDA US. Guidance for industry: dissolution testing of immediate-release solid oral dosage forms. Food and Drug Administration, Center for Drug Evaluation and Research(CDER). 1997.
Gonzalez-Garcia I, Mangas-Sanjuan V, Merino-Sanjuan M, Alvarez-Alvarez C, Diaz-Garzon Marco J, Rodriguez-Bonnin MA, et al. IVIVC approach based on carbamazepine bioequivalence studies combination. Pharmazie. 2017;72(8):449–55. https://doi.org/10.1691/ph.2017.7011.
Kim TH, Bulitta JB, Kim DH, Shin S, Shin BS. Novel extended in vitro-in vivo correlation model for the development of extended-release formulations for baclofen: from formulation composition to in vivo pharmacokinetics. Int J Pharm. 2019;556:276–86. https://doi.org/10.1016/j.ijpharm.2018.12.007.
Tietz K, Gutknecht SI, Klein S. Predicting local drug availability of locally acting lozenges: from method design to a linear level A IVIVC. Eur J Pharm Biopharm. 2018;133:269–76. https://doi.org/10.1016/j.ejpb.2018.10.015.
Jacob S, Nair AB. An updated overview with simple and practical approach for developing in vitro-in vivo correlation. Drug Dev Res. 2018;79(3):97–110. https://doi.org/10.1002/ddr.21427.
Kataoka M, Yano K, Hamatsu Y, Masaoka Y, Sakuma S, Yamashita S. Assessment of absorption potential of poorly water-soluble drugs by using the dissolution/permeation system. Eur J Pharm Biopharm. 2013;85(3 Pt B):1317–24. https://doi.org/10.1016/j.ejpb.2013.06.018.
Higuchi M, Nishida S, Yoshihashi Y, Tarada K, Sugano K. Prediction of coning phenomena for irregular particles in paddle dissolution test. Eur J Pharm Sci. 2015;76:213–6. https://doi.org/10.1016/j.ejps.2015.05.019.
Gray VA. Pharmaceutical analysis | dissolution testing. In: Worsfold P, Poole C, Townshend A, Miró M, editors. Encyclopedia of analytical science. Third ed. Oxford: Academic Press; 2019. p. 182–7.
Hate SS, Reutzel-Edens SM, Taylor LS. Absorptive dissolution testing of supersaturating systems: impact of absorptive sink conditions on solution phase behavior and mass transport. Mol Pharm. 2017;14(11):4052–63. https://doi.org/10.1021/acs.molpharmaceut.7b00740.
Delalonde M, Ruiz T. Dissolution of pharmaceutical tablets: the influence of penetration and drainage of interstitial fluids. Chem Eng Process. 2008;47(3):370–6. https://doi.org/10.1016/j.cep.2007.01.003.
Park K. Absence of in vivo-in vitro correlation in per-oral drug delivery. J Control Release. 2014;180:150. https://doi.org/10.1016/j.jconrel.2014.03.020.
Lee C-M, Luner PE, Locke K, Briggs K. Application of an artificial stomach-duodenum reduced gastric pH dog model for formulation principle assessment and mechanistic performance understanding. J Pharm Sci. 2017;106(8):1987–97. https://doi.org/10.1016/j.xphs.2017.02.015.
Mizoguchi M, Kataoka M, Yokoyama K, Aihara R, Wada K, Yamashita S. Application of an in vitro dissolution/permeation system to early screening of oral formulations of poorly soluble, weakly basic drugs containing an acidic pH-modifier. J Pharm Sci. 2018;107(9):2404–10. https://doi.org/10.1016/j.xphs.2018.05.009.
Shi Y, Gao P, Gong Y, Ping H. Application of a biphasic test for characterization of in vitro drug release of immediate release formulations of celecoxib and its relevance to in vivo absorption. Mol Pharm. 2010;7(5):1458–65. https://doi.org/10.1021/mp100114a.
Phillips DJ, Pygall SR, Cooper VB, Mann JC. Overcoming sink limitations in dissolution testing: a review of traditional methods and the potential utility of biphasic systems. J Pharm Pharmacol. 2012;64(11):1549–59. https://doi.org/10.1111/j.2042-7158.2012.01523.x.
Fotaki N, Symillides M, Reppas C. In vitro versus canine data for predicting input profiles of isosorbide-5-mononitrate from oral extended release products on a confidence interval basis. Eur J Pharm Sci. 2005;24(1):115–22. https://doi.org/10.1016/j.ejps.2004.10.003.
Shono Y, Jantratid E, Janssen N, Kesisoglou F, Mao Y, Vertzoni M, et al. Prediction of food effects on the absorption of celecoxib based on biorelevant dissolution testing coupled with physiologically based pharmacokinetic modeling. Eur J Pharm Biopharm. 2009;73(1):107–14. https://doi.org/10.1016/j.ejpb.2009.05.009.
Yazdanian M, Briggs K, Jankovsky C, Hawi A. The “high solubility” definition of the current FDA Guidance on Biopharmaceutical Classification System may be too strict for acidic drugs. Pharm Res. 2004;21(2):293–9.
Gangadharappa HV, Chandra Prasad SM, Singh RP. Formulation, in vitro and in vivo evaluation of celecoxib nanosponge hydrogels for topical application. J Drug Deliv Sci Technol. 2017;41:488–501. https://doi.org/10.1016/j.jddst.2017.09.004.
Mudie DM, Murray K, Hoad CL, Pritchard SE, Garnett MC, Amidon GL, et al. Quantification of gastrointestinal liquid volumes and distribution following a 240 mL dose of water in the fasted state. Mol Pharm. 2014;11(9):3039–47. https://doi.org/10.1021/mp500210c.
Kostewicz ES, Abrahamsson B, Brewster M, Brouwers J, Butler J, Carlert S, et al. In vitro models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57:342–66. https://doi.org/10.1016/j.ejps.2013.08.024.
Kakhi M. Classification of the flow regimes in the flow-through cell. Eur J Pharm Sci. 2009;37(5):531–44. https://doi.org/10.1016/j.ejps.2009.04.003.
Wagner JG, Nelson E. Kinetic analysis of blood levels and urinary excretion in the absorptive phase after single doses of drug. J Pharm Sci. 1964;53(11):1392–403. https://doi.org/10.1002/jps.2600531126.
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, et al. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12(3):263–71. https://doi.org/10.1208/s12248-010-9185-1.
Ruiz Picazo A, Martinez-Martinez MT, Colon-Useche S, Iriarte R, Sanchez-Dengra B, Gonzalez-Alvarez M, et al. In vitro dissolution as a tool for formulation selection: Telmisartan two-step IVIVC. Mol Pharm. 2018;15(6):2307–15. https://doi.org/10.1021/acs.molpharmaceut.8b00153.
Asmanova N, Koloskov G, Ilin AI. Coupled solutions of one- and two-compartment pharmacokinetic models with first-order absorption. J Pharmacokinet Pharmacodyn. 2013;40(2):229–41. https://doi.org/10.1007/s10928-013-9312-6.
EMA. Guideline on quality of oral modified release products. 2014.
FDA. Guidance for industry: extended release oral dosage forms: development, evaluation and application of in vitro/in vivo correlations. Food and Drug Administration, Rockville, MD. 1997.
Misra S, Wahab MF, Patel DC, Armstrong DW. The utility of statistical moments in chromatography using trapezoidal and Simpson’s rules of peak integration. J Sep Sci. 2019;42(8):1644–57. https://doi.org/10.1002/jssc.201801131.
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33.
Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods. 2012;17(2):228–43. https://doi.org/10.1037/a0027127.
Nicolaides E, Galia E, Efthymiopoulos C, Dressman JB, Reppas C. Forecasting the in vivo performance of four low solubility drugs from their in vitro dissolution data. Pharm Res. 1999;16(12):1876–82. https://doi.org/10.1023/a:1018959511323.
Dressman JB, Reppas C. In vitro-in vivo correlations for lipophilic, poorly water-soluble drugs. Eur J Pharm Sci. 2000;11(Suppl 2):S73–80.
Posti J, Speiser PP. Sink conditions in the flow-through cell during dissolution. Int J Pharm. 1980;5:101–7.
Wang F, Barnes TJ, Prestidge CA. Celecoxib confinement within mesoporous silicon for enhanced oral bioavailability. Mesoporous Biomaterials. 2014;1(1). https://doi.org/10.2478/mesbi-2013-0001.
Paulson SK, Hribar JD, Liu NW, Hajdu E, et al. Metabolism and excretion of [(14)C] celecoxib in healthy male volunteers. Drug Metab Dispos. 2000;28:308–14.
Levy G, Leonards JR, Procknal JA. Interpretation of in vitro dissolution data relative to the gastrointestinal absorption characteristics of drugs in tablets. J Pharm Sci. 1967;56(10):1365–7. https://doi.org/10.1002/jps.2600561039.
Klein S, Shah VP. A standardized mini paddle apparatus as an alternative to the standard paddle. AAPS PharmSciTech. 2008;9(4):1179–84. https://doi.org/10.1208/s12249-008-9161-6.
Schall R, Luus HG, Steinijans VW, Hauschke D. Choice of characteristics and their bioequivalence ranges for the comparison of absorption rates of immediate-release drug formulations. Int J Clin Pharmacol Ther. 1994;32(7):323–8.
Kakhi M, Suarez-Sharp S, Shepard T, Chittenden J. Application of an NLME–stochastic deconvolution approach to level A IVIVC modeling. J Pharm Sci. 2017;106(7):1905–16. https://doi.org/10.1016/j.xphs.2017.03.015.
Li ZQ, He X, Gao X, Xu YY, Wang YF, Gu H, et al. Study on dissolution and absorption of four dosage forms of isosorbide mononitrate: level A in vitro-in vivo correlation. Eur J Pharm Biopharm. 2011;79(2):364–71. https://doi.org/10.1016/j.ejpb.2011.04.015.
Cupera J, Lansky P, Sklubalova Z. Sampling times influence the estimate of parameters in the Weibull dissolution model. Eur J Pharm Sci. 2015;78:171–6. https://doi.org/10.1016/j.ejps.2015.07.015.
Cardot JM, Lukas JC, Muniz P. Time scaling for in vitro-in vivo correlation: the inverse release function (IRF) approach. AAPS J. 2018;20(6):95. https://doi.org/10.1208/s12248-018-0250-5.
Park MS, Shim WS, Yim SV, Lee KT. Development of simple and rapid LC-MS/MS method for determination of celecoxib in human plasma and its application to bioequivalence study. J Chromatogr B. 2012;902:137–41. https://doi.org/10.1016/j.jchromb.2012.06.016.
Paulson SK, Vaughn MB, Jessen SM, Lawal Y, Gresk CJ, Yan B, et al. Pharmacokinetics of celecoxib after oral administration in dogs and humans: effect of food and site of absorption. J Pharmacol Exp Ther. 2001;297(2):638–45.
Acknowledgments
This research was supported by Hunan Huize Bio-pharmaceutical Co., Ltd., and Hunan Taixin Pharmaceutical Technology Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 12 kb).
Rights and permissions
About this article
Cite this article
Jiang, S., Zhang, G., Wang, L. et al. Development of a Two-Compartment System In vitro Dissolution Test and Correlation with In vivo Pharmacokinetic Studies for Celecoxib. AAPS PharmSciTech 21, 59 (2020). https://doi.org/10.1208/s12249-019-1612-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1612-8