Abstract
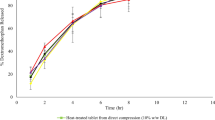
Patients who suffer from dysphagia have difficulty in swallowing hard tablets and capsules; hence, gelatin-based soft-chew dosages are used as an alternative and novel drug delivery approach to overcome this problem. However, the conventional method of producing gelatin-based soft-chew dosages has many potential issues. The objective of this study was to use glycerol and the hot-melt extrusion technique to address potential issues and optimize the formulation. Gelatin, acetaminophen, saccharin, xylitol, and sodium chloride and six different ratios of water and glycerol were used in the seven formulations. Extrusion process temperature of formulations 1–6 and formulation 7 were 90°C and 140°C, respectively. Near-infrared spectra were collected during extrusion to monitor quality consistency. Scanning electron microscopic images of the cross-section of the soft-chew dosages were recorded. Differential scanning calorimetry (DSC) was used to characterize the crystal states of each formulation. Texture profile analysis was used to evaluate the physical properties of the tablets. In vitro drug release characteristics were studied. A 45-day stability study was carried out to evaluate the stability of each formulation. Near-infrared spectra showed that formulations 1–6 were uniform while formulation 7 was not. From the DSC results, formulations 1 and 2 showed crystallinity of acetaminophen. Formulation 5 displayed the desired physical and chemical stability in texture profile analysis and in the in vitro drug release studies. By using glycerol and hot-melt extrusion, the potential issues of conventional methods were successfully addressed.









Similar content being viewed by others
References
Aulton ME, Taylor K. Aulton’s pharmaceutics: the design and manufacture of medicines. Churchill Livingstone/Elsevier; 2013. 894 p.
Liu F, Ranmal S, Batchelor HK, Orlu-Gul M, Ernest TB, Thomas IW, et al. Patient-centered pharmaceutical design to improve acceptability of medicines: similarities and differences in paediatric and geriatric populations. Drugs. 2014 Oct 2;74(16):1871–89.
Dille MJ, Hattrem MN, Draget KI. Soft, chewable gelatin-based pharmaceutical oral formulations: a technical approach. Pharm Dev Technol. 2017 Jun;2:1–8.
Manmohan T, Sreenivas G, Sastry VV, Sudha RE, Indira K, Ushasree T, et al. Drug compliance and adherence to treatment. J Evol Med Dent Sci.
Karim AA, Bhat R. Gelatin alternatives for the food industry: recent developments, challenges and prospects. Trends Food Sci Technol. 2008 Dec 1;19(12):644–56.
Morrison NA, Clark RC, Chen YL, Talashek T, Sworn G. Gelatin alternatives for the food industry. In: Physical chemistry and industrial application of gellan gum. Berlin, Heidelberg: Springer Berlin Heidelberg; 1999. p. 127–31.
Djagny KB, Wang Z, Xu S. Gelatin: a valuable protein for food and pharmaceutical industries: review. Crit Rev Food Sci Nutr. 2001 Nov;41(6):481–92.
Ruheena T, Sirisha M. Soft chewable drug delivery system: oral medicated jelly and soft chew. J Drug Deliv Ther. 2018;8(4):65–72.
Kavoosi G, Dadfar SMM, Purfard AM. Mechanical, physical, antioxidant, and antimicrobial properties of gelatin films incorporated with thymol for potential use as nano wound dressing. J Food Sci. 2013 Feb 1;78(2):E244–50.
Carmona A, Freedland RA. Effect of glycerol and dihydroxyacetone on hepatic lipogenesis. Arch Biochem Biophys. 1989 May 15;271(1):130–8.
Pisani DF, Bottema CDK, Butori C, Dani C, Dechesne CA. Mouse model of skeletal muscle adiposity: a glycerol treatment approach. Biochem Biophys Res Commun. 2010 Jun 4;396(3):767–73.
Bartsch W, Sponer G, Dietmann K, Fuchs G. Acute toxicity of various solvents in the mouse and rat. LD50 of ethanol, diethylacetamide, dimethylformamide, dimethylsulfoxide, glycerine, N-methylpyrrolidone, polyethylene glycol 400, 1,2-propanediol and Tween 20. Arzneimittelforschung. 1976;26(8):1581–3.
HINE CH, ANDERSON HH, MOON HD, DUNLAP MK, MORSE MS. Comparative toxicity of synthetic and natural glycerin. AMA Arch Ind Hyg Occup Med. 1953 Apr;7(4):282–91.
References 1, Merck, Index E, Budavari S, Ed. Product information of gelatin. J Immunol Methods. 1996;742(43):4388–1017.
Pagliaro M, Rossi M. The future of glycerol [internet]. Cambridge: Royal Society of Chemistry; 2008. (Green Chemistry Series).
Timasheff SN. Control of protein stability and reactions by weakly interacting cosolvents: the simplicity of the complicated. Adv Protein Chem. 1998;51(51):355–432.
Douroumis D. Hot-melt extrusion : pharmaceutical applications [internet]. Wiley; 2012.
Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Kumar Battu S, et al. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33(9):909–26.
MISHRA B, SHARMA G, SHUKLA D. Investigation of organoleptic characteristics in the development of soft chews of calcium carbonate as mineral supplement. Yakugaku Zasshi. 2009;129(12):1537–44.
Bourne MC. Food texture and viscosity: concept and measurement academic. N Y. 1982.
Raadsheer MC, van Eijden TMGJ, van Ginkel FC, Prahl-Andersen B. Contribution of jaw muscle size and craniofacial morphology to human bite force magnitude. J Dent Res. 1999 Jan 8;78(1):31–42.
Morsy R, Hosny M, Reicha F, Elnimr T. Developing and physicochemical evaluation of cross-linked electrospun gelatin–glycerol nanofibrous membranes for medical applications. J Mol Struct. 2017 May 5;1135:222–7.
Sanwlani S, Kumar P, Bohidar HB. Hydration of gelatin molecules in glycerol-water solvent and phase diagram of gelatin organogels. J Phys Chem B. 2011 Jun 9;115(22):7332–40.
Acknowledgments
The authors thank the Pii Center for Pharmaceutical Innovation & Instruction and Grant Number P30GM122733-01A1, funded by the National Institute of General Medical Sciences (NIGMS), a component of the National Institutes of Health (NIH) as one of its Centers of Biomedical Research Excellence (COBRE). We are thankful to ThermoFisher Scientific for their help and advice to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, P., Zhang, J., Bandari, S. et al. A Novel Acetaminophen Soft-Chew Formulation Produced via Hot-Melt Extrusion with In-line Near-Infrared Monitoring as a Process Analytical Technology Tool. AAPS PharmSciTech 21, 37 (2020). https://doi.org/10.1208/s12249-019-1596-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1596-4