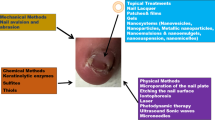
Abstract
Onychomycosis is a chronic disorder that is difficult to manage and hard to eradicate with perilous trends to relapse. Due to increased prevalence of HIV, use of immunosuppressant drugs and lifestyle-related factors, population affected with fungal infection of nail (Onychomycosis) happens to increase extensively in last two decades. Modalities available for the treatment of onychomycosis include systemically administered antifungals, mechanical procedures, and topical drug therapy. But the efficacy of the most of approaches to deliver drug at targeted site, i.e., deep-seated infected nail bed is limited due to compact and highly keratinized nail structure. A series of advanced formulation approaches, such as transfersomes, liposomes, nano/micro emulsion, nail lacquers etc., have been attempted to improve the drug penetration into nail plate more efficiently. The manuscript reviews these formulation approaches with their possible mechanisms by which they improve the drug penetration.Comparative analysis of available treatment modalities for onychomycosis has been provided with pros and cons of each alternatives. Additionally, ongoing research about the application of biological materials such as modified cationic antimicrobial peptides (AMPs), plant-derived proteins, and synthetic antimicrobial peptidomimetics have also been explored.



Similar content being viewed by others
References
Murdan S. Drug delivery to the nail following topical application. Int J Pharm. 2002;236(1–2):1–26.
Hossain MA, Ghannoum MA. New developments in chemotherapy for non-invasive fungal infections. Expert Opin Investig Drugs. 2001;10(8):1501–11.
Gupta AK, Jain HC, Lynde CW, MacDonald P, Cooper EA, Summerbell RC. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43(2):244–8.
Ahmadi B, Hashemi SJ, Zaini F, Shidfar MR, Moazeni M, Mousavi B, et al. A case of onychomycosis caused by Aspergillus candidus. Medical mycology case reports. 2012;1(1):45–8.
Tiwary AK, Sapra B. High failure rate of transungal drug delivery: need for new strategies. Ther Deliv. 2017;8(5):239–42.
Zaias N. Onychomycosis. Dermatol Clin. 1985;3(3):445–60.
Baran R, Hay R, Tosti A, Haneke E. A new classification of onychomycosis. Br J Dermatol. 1998;139(4):567–71.
Baran R. Proximal subungual candida onychomycosis. An unusual manifestation of chronic muco-cutaneous candidosis. Br J Dermatol. 1997;137(2):286–8.
Baran R, Tosti A, Piraccini B. Uncommon clinical patterns of Fusarium nail infection: report of three cases. Br J Dermatol. 1997;136(3):424–7.
Tosti A, Piraccini B, Stinchi C, Lorenzi S. Onychomycosis due to Scopulariopsis brevicaulis: clinical features and response to systemic antifungals. Br J Dermatol. 1996;135(5):799–802.
Hay R, Baran R, Moore M, Wilkinson J. Candida onychomycosis—an evaluation of the role of Candida species in nail disease. Br J Dermatol. 1988;118(1):47–58.
Gupta AK, Simpson FC. New therapeutic options for onychomycosis. Expert Opin Pharmacother. 2012;13(8):1131–42.
Krishnan-Natesan S. Terbinafine: a pharmacological and clinical review. Expert Opin Pharmacother. 2009;10(16):2723–33.
Ghannoum MA, Long L, Pfister WR. Determination of the efficacy of terbinafine hydrochloride nail solution in the topical treatment of dermatophytosis in a Guinea pig model. Mycoses. 2009;52(1):35–43.
Girmenia C. New generation azole antifungals in clinical investigation. Expert Opin Investig Drugs. 2009;18(9):1279–95.
Baker SJ, Zhang Y-K, Akama T, Lau A, Zhou H, Hernandez V, et al. Discovery of a new boron-containing antifungal agent, 5-fluoro-1, 3-dihydro-1-hydroxy-2, 1-benzoxaborole (AN2690), for the potential treatment of onychomycosis. J Med Chem. 2006;49(15):4447–50.
Gupta AK, Studholme C. Novel investigational therapies for onychomycosis: an update. Expert Opin Investig Drugs. 2016;25(3):297–305.
Hui X, Baker SJ, Wester RC, Barbadillo S, Cashmore AK, Sanders V, et al. In vitro penetration of a novel oxaborole antifungal (AN2690) into the human nail plate. J Pharm Sci. 2007;96(10):2622–31.
Beutner K, Toledo-Bahena M, Barbosa-Alanis H, Reyes-Santana E, Mata-Lara MG, Santillán ALL-T, et al. Interim results of a multi-center study to evaluate the safety and efficacy of topically applied AN2690 5.0% and 7.5% solutions for the treatment of onychomycosis of the great toe nail. J Am Acad Dermatol. 2007;56(2).
Jinna S, Finch J. Spotlight on tavaborole for the treatment of onychomycosis. Drug design, development and therapy. 2015;9:6185.
Rock FL, Mao W, Yaremchuk A, Tukalo M, Crépin T, Zhou H, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316(5832):1759–61.
Gupta AK, Simpson FC. New pharmacotherapy for the treatment of onychomycosis: an update. Expert Opin Pharmacother. 2015;16(2):227–36.
Scher R, Baran R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149:5–9.
Arrese JE, Piérard GE. Treatment failures and relapses in onychomycosis: a stubborn clinical problem. Dermatology. 2003;207(3):255–60.
Shivakumar H, Juluri A, Desai B, Murthy SN. Ungual and transungual drug delivery. Drug Dev Ind Pharm. 2012;38(8):901–11.
Fonzo D. L.I.ON. Study: efficacy and tolerability of continuous terbinafine (Lamisil®) compared to intermittent itraconazole in the treatment of toenail onychomycosis. Br J Dermatol. 1999;141:5–14.
Sigurgeirsson B, Ólafsson JH, Steinsson J, Paul C, Billstein S, EGV E. Long-term effectiveness of treatment with terbinafine vs itraconazole in onychomycosis: a 5-year blinded prospective follow-up study. Arch Dermatol. 2002;138(3):353–7.
Villars V, Jones T. Special features of the clinical use of oral terbinafine in the treatment of fungal diseases. Br J Dermatol. 1992;126:61–9.
Tosti A, Piraccini B, Stinchi C, Colombo M. Relapses of onychomycosis after successful treatment with systemic antifungals: a three-year follow-up. Dermatology. 1998;197(2):162–6.
Baran RMaibach H. Textbook of cosmetic dermatology. London Crossref: Informa Healthcare; 2010.
Piraccini BM, Rech G, Tosti A. Photodynamic therapy of onychomycosis caused by Trichophyton rubrum. J Am Acad Dermatol. 2008;59(5):S75–S6.
Nair AB, Kim HD, Chakraborty B, Singh J, Zaman M, Gupta A, et al. Ungual and trans-ungual iontophoretic delivery of terbinafine for the treatment of onychomycosis. J Pharm Sci. 2009;98(11):4130–40.
Boker A, Bea YS, Gowrishankar TR, Ciocon D, Kimball AB. A double-blind, placebo-controlled, pilot study of 1% terbinafine cream delivered via toenail microconduits for the treatment of subungual onychomycosis. Poster presented at the 65th annual meeting of the American Academy of Dermatology, Washington, DC; 2007.
Ciocon D, Gowrishankar T, Herndon T, Kimball AB. How low should you go: novel device for nail trephination. Dermatol Surg. 2006;32(6):828–33.
Murdan S. Enhancing the nail permeability of topically applied drugs. Expert opinion on drug delivery. 2008;5(11):1267–82.
Mohorčič M, Torkar A, Friedrich J, Kristl J, Murdan S. An investigation into keratinolytic enzymes to enhance ungual drug delivery. Int J Pharm. 2007;332(1–2):196–201.
Miron D, Cornelio R, Troleis J, Mariath J, Zimmer A, Mayorga P, et al. Influence of penetration enhancers and molecular weight in antifungals permeation through bovine hoof membranes and prediction of efficacy in human nails. Eur J Pharm Sci. 2014;51:20–5.
Amichai B, Mosckovitz R, Trau H, Sholto O, Ben-Yaakov S, Royz M, et al. Iontophoretic terbinafine HCL 1.0% delivery across porcine and human nails. Mycopathologia. 2010;169(5):343–9.
Monti D, Egiziano E, Burgalassi S, Tampucci S, Terreni E, Tivegna S, et al. Influence of a combination of chemical enhancers and Iontophoresis on in vitro Transungual permeation of Nystatin. AAPS PharmSciTech. 2018;19(4):1574–81.
Nair AB, Singh K, Shinu P, Harsha S, Al-Dhubiab BE. A comprehensive study to evaluate the effect of constant low voltage iontophoresis on transungual delivery. Drug Dev Ind Pharm. 2013;39(5):807–15.
Vanstone S, Cordery SF, Stone JM, Gordeev SN, Guy RH. Precise laser poration to control drug delivery into and through human nail. J Control Release. 2017;268:72–7.
Salter SA, Ciocon DH, Gowrishankar TR, Kimball AB. Controlled nail trephination for subungual hematoma. Am J Emerg Med. 2006;24(7):875–7.
Gupta AK, FRCP. Ciclopirox: an overview. Int J Dermatol. 2001;40(5):305–10.
Gupta AK, Plott T. Ciclopirox: a broad-spectrum antifungal with antibacterial and anti-inflammatory properties. Int J Dermatol. 2004;43(S1):3–8.
Polak A. Mode of action of morpholine derivatives. Ann N Y Acad Sci. 1988;544(1):221–8.
Alley MR, Baker SJ, Beutner KR, Plattner J. Recent progress on the topical therapy of onychomycosis. Expert Opin Investig Drugs. 2007;16(2):157–67.
Thatai P, Sapra B. Transungual delivery: deliberations and creeds. Int J Cosmet Sci. 2014;36(5):398–411.
Kobayashi Y, Miyamoto M, Sugibayashi K, Morimoto Y. Drug permeation through the three layers of the human nail plate. J Pharm Pharmacol. 1999;51(3):271–8.
Walters KA, Flynn GL, Marvel JR. Physicochemical characterization of the human nail: permeation pattern for water and the homologous alcohols and differences with respect to the stratum corneum. J Pharm Pharmacol. 1983;35(1):28–33.
Walters K, Flynn G. Permeability characteristics of the human nail plate. Int J Cosmet Sci. 1983;5(6):231–46.
Garson J, Baltenneck F, Leroy F, Riekel C, Müller M. Histological structure of human nail as studied by synchrotron X-ray microdiffraction. Cellular and molecular biology (Noisy-le-Grand, France). 2000;46(6):1025–34.
Forslind B. Biophysical studies of the normal nail. Acta Derm Venereol. 1970;50(3):161–8.
Vejnovic I, Simmler L, Betz G. Investigation of different formulations for drug delivery through the nail plate. Int J Pharm. 2010;386(1–2):185–94.
Akhtar N, Sharma H, Pathak K. Onychomycosis: potential of nail lacquers in transungual delivery of antifungals. Scientifica. 2016;2016(1387936):12.
Khengar R, Jones S, Turner R, Forbes B, Brown M. Nail swelling as a pre-formulation screen for the selection and optimisation of ungual penetration enhancers. Pharm Res. 2007;24(12):2207–12.
Smith KA, Hao J, Li SK. Effects of organic solvents on the barrier properties of human nail. J Pharm Sci. 2011;100(10):4244–57.
Monti D, Saccomani L, Chetoni P, Burgalassi S, Saettone M, Mailland F. In vitro transungual permeation of ciclopirox from a hydroxypropyl chitosan-based, water-soluble nail lacquer. Drug Dev Ind Pharm. 2005;31(1):11–7.
Elsayed MM. Development of topical therapeutics for management of onychomycosis and other nail disorders: a pharmaceutical perspective. J Control Release. 2015;199:132–44.
Shivakumar H, Vaka SRK, Madhav NS, Chandra H, Murthy SN. Bilayered nail lacquer of terbinafine hydrochloride for treatment of onychomycosis. J Pharm Sci. 2010;99(10):4267–76.
Thatai P, Sapra B. Terbinafine hydrochloride nail lacquer for the management of onychomycosis: formulation, characterization and in vitro evaluation. Ther Deliv. 2018;9(2):99–119.
Šveikauskaitė I, Briedis V. Effect of film-forming polymers on release of naftifine hydrochloride from nail lacquers. Int J of Poly Sci. 2017;2017(1476270):7.
Cutrín-Gómez E, Anguiano-Igea S, Delgado-Charro M, Gómez-Amoza J, Otero-Espinar F. Effect on nail structure and Transungual permeability of the ethanol and Poloxamer ratio from Cyclodextrin-soluble Polypseudorotaxanes based nail lacquer. Pharmaceutics. 2018;10(3):156.
Joshi M, Sharma V, Pathak K. Matrix based system of isotretinoin as nail lacquer to enhance transungal delivery across human nail plate. Int J Pharm. 2015;478(1):268–77.
Cutrín-Gómez E, Anguiano-Igea S, Delgado-Charro M, Gómez-Amoza J, Otero-Espinar F. Effect of penetration enhancers on drug nail permeability from Cyclodextrin/Poloxamer-soluble Polypseudorotaxane-based nail lacquers. Pharmaceutics. 2018;10(4):273.
Thapa RK, Choi JY, Go TG, Kang MH, Han SD, Jun J-H, et al. Development of ciclopirox nail lacquer with enhanced permeation and retention. Arch Pharm Res. 2016;39(7):953–9.
Khattab A, Shalaby S. Optimized Ciclopirox-based Eudragit RLPO nail lacquer: effect of Endopeptidase enzyme as permeation enhancer on Transungual drug delivery and efficiency against Onychomycosis. AAPS PharmSciTech. 2018;19(3):1048–60.
El-sherif NI, Shamma RN, Abdelbary G. In-situ gels and nail lacquers as potential delivery systems for treatment of onychomycosis. A comparative study. Journal of Drug Delivery Science and Technology. 2018;43:253–61.
Valdes BSG, Serro AP, Gordo PM, Silva A, Gonçalves L, Salgado A, et al. New polyurethane nail lacquers for the delivery of Terbinafine: formulation and antifungal activity evaluation. J Pharm Sci. 2017;106(6):1570–7.
Shah VH, Jobanputra A. Enhanced ungual permeation of terbinafine HCl delivered through liposome-loaded nail lacquer formulation optimized by QbD approach. AAPS PharmSciTech. 2018;19(1):213–24.
Nogueiras-Nieto L, Delgado-Charro MB, Otero-Espinar FJ. Thermogelling hydrogels of cyclodextrin/poloxamer polypseudorotaxanes as aqueous-based nail lacquers: application to the delivery of triamcinolone acetonide and ciclopirox olamine. Eur J Pharm Biopharm. 2013;83(3):370–7.
Hafeez F, Hui X, Chiang A, Hornby S, Maibach H. Transungual delivery of ketoconazole using novel lacquer formulation. Int J Pharm. 2013;456(2):357–61.
Sipponen P, Sipponen A, Lohi J, Soini M, Tapanainen R, Jokinen JJ. Natural coniferous resin lacquer in treatment of toenail onychomycosis: an observational study. Mycoses. 2013;56(3):289–96.
Hassan N, Singh M, Sulaiman S, Jain P, Sharma K, Nandy S, et al. Molecular docking-guided Ungual drug-delivery Design for Amelioration of Onychomycosis. ACS Omega. 2019;4(5):9583–92.
Thatai P, Kaur K, Sapra B. In-vitro evaluation of Transungual formulation of ketoconazole for the Management of Onychomycosis. Drug Delivery Letters. 2018;8(2):140–52.
Hui X, Chan TC, Barbadillo S, Lee C, Maibach HI, Wester RC. Enhanced econazole penetration into human nail by 2-n-nonyl-1, 3-dioxolane. J Pharm Sci. 2003;92(1):142–8.
Bhuptani RS, Deshpande KM, Patravale VB. Transungual permeation: current insights. Drug delivery and translational research. 2016;6(4):426–39.
Myoung Y, Choi H-K. Permeation of ciclopirox across porcine hoof membrane: effect of pressure sensitive adhesives and vehicles. Eur J Pharm Sci. 2003;20(3):319–25.
Donnelly RF, McCarron PA, Lightowler JM, Woolfson AD. Bioadhesive patch-based delivery of 5-aminolevulinic acid to the nail for photodynamic therapy of onychomycosis. J Control Release. 2005;103(2):381–92.
Mididoddi PK, Repka MA. Characterization of hot-melt extruded drug delivery systems for onychomycosis. Eur J Pharm Biopharm. 2007;66(1):95–105.
Mididoddi P, Prodduturi S, Repka M. Influence of tartaric acid on the bioadhesion and mechanical properties of hot-melt extruded hydroxypropyl cellulose films for the human nail. Drug Dev Ind Pharm. 2006;32(9):1059–66.
Repka MA, Mididoddi PK, Stodghill SP. Influence of human nail etching for the assessment of topical onychomycosis therapies. Int J Pharm. 2004;282(1–2):95–106.
Touitou E. Drug delivery across the skin. Expert Opin Biol Ther. 2002;2(7):723–33.
Ghannoum M, Isham N, Herbert J, Henry W, Yurdakul S. Activity of TDT 067 (terbinafine in Transfersome) against agents of onychomycosis, as determined by minimum inhibitory and fungicidal concentrations. J Clin Microbiol. 2011;49(5):1716–20.
Sigurgeirsson B, Ghannoum M. Therapeutic potential of TDT 067 (terbinafine in Transfersome®): a carrier-based dosage form of terbinafine for onychomycosis. Expert Opin Investig Drugs. 2012;21(10):1549–62.
Naumann S, Meyer J-P, Kiesow A, Mrestani Y, Wohlrab J, Neubert RH. Controlled nail delivery of a novel lipophilic antifungal agent using various modern drug carrier systems as well as in vitro and ex vivo model systems. J Control Release. 2014;180:60–70.
Pannu J, McCarthy A, Martin A, Hamouda T, Ciotti S, Fothergill A, et al. NB-002, a novel nanoemulsion with broad antifungal activity against dermatophytes, other filamentous fungi, and Candida albicans. Antimicrob Agents Chemother. 2009;53(8):3273–9.
Barot BS, Parejiya PB, Patel HK, Gohel MC, Shelat PK. Microemulsion-based gel of terbinafine for the treatment of onychomycosis: optimization of formulation using D-optimal design. AAPS PharmSciTech. 2012;13(1):184–92.
Mahtab A, Anwar M, Mallick N, Naz Z, Jain GK, Ahmad FJ. Transungual delivery of ketoconazole nanoemulgel for the effective management of onychomycosis. AAPS PharmSciTech. 2016;17(6):1477–90.
Amra K, Momin M. Formulation evaluation of ketoconazole microemulsion-loaded hydrogel with nigella oil as a penetration enhancer. J Cosmet Dermatol. 2019.
Tanrıverdi ST, Özer Ö. Novel topical formulations of Terbinafine-HCl for treatment of onychomycosis. Eur J Pharm Sci. 2013;48(4–5):628–36.
Tuncay Tanrıverdi S, Hilmioğlu Polat S, Yeşim Metin D, Kandiloğlu G, Özer Ö. Terbinafine hydrochloride loaded liposome film formulation for treatment of onychomycosis: in vitro and in vivo evaluation. Journal of liposome research. 2016;26(2):163–73.
Dhamoon RK, Popli H, Gupta M. Novel drug delivery strategies for the treatment of Onychomycosis. Pharmaceutical nanotechnology. 2019;7(1):24–38.
Bseiso EA, Nasr M, Sammour OA. Abd El Gawad NA. Novel nail penetration enhancer containing vesicles “nPEVs” for treatment of onychomycosis. Drug delivery. 2016;23(8):2813–9.
Elsherif NI, Shamma RN, Abdelbary G. Terbinafine hydrochloride trans-ungual delivery via nanovesicular systems: in vitro characterization and ex vivo evaluation. AAPS PharmSciTech. 2017;18(2):551–62.
Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55(1):27–55.
Isaksson J, Brandsdal BO, Engqvist M, Flaten GE, Svendsen JSM, Stensen W. A synthetic antimicrobial peptidomimetic (LTX 109): stereochemical impact on membrane disruption. J Med Chem. 2011;54(16):5786–95.
Strøm MB, Haug BE, Skar ML, Stensen W, Stiberg T, Svendsen JS. The pharmacophore of short cationic antibacterial peptides. J Med Chem. 2003;46(9):1567–70.
Haug BE, Stensen W, Kalaaji M, Rekdal Ø, Svendsen JS. Synthetic antimicrobial peptidomimetics with therapeutic potential. J Med Chem. 2008;51(14):4306–14.
Stensen W, Turner R, Brown M, Kondori N, Svendsen JS, Svenson J. Short cationic antimicrobial peptides display superior antifungal activities toward candidiasis and Onychomycosis in comparison with Terbinafine and Amorolfine. Mol Pharm. 2016;13(10):3595–600.
Chaudhuri B, Chim MF, Bucks D. Topical formulations for the treatment of nail fungal diseases. Int Patent App. 1999:28.
Quan D, Ruiz A. Nail compositions and methods of administering same. Int Patent App. 2002:28.
Maibach HI, Luo EC, Hsu TM. Topical administration of basic antifungal compositions to treat fungal infections of the nails. U.S. Patent. 2005:13.
Riley L. Topical formulation for treating fingernails and toenails. U.S. Patent. 2007:4.
Kepka SW, Mo YJ, Wang HY, Lu M, Pfister WR, inventors; Google Patents, assignee. Antifungal nail coat and method of use2008.
Chakraborty B, Barsness MS, Goldberg DI, Etheredge RW, Davis SP. Method and system for treating of onychomycosis with an applicator having a gel medicament layer. Int Patent App. 2008:49.
Bailey C. Method and apparatus for improving the appearance of nails affected by onychomycosis through the topical application of highly concentrated or supersaturated boric acid. U.S. Patent App. 2011:10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vikas, A., Rashmin, P., Mrunali, P. et al. Mechanistic Insights of Formulation Approaches for the Treatment of Nail Infection: Conventional and Novel Drug Delivery Approaches. AAPS PharmSciTech 21, 67 (2020). https://doi.org/10.1208/s12249-019-1591-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1591-9