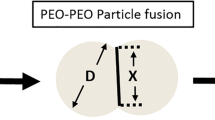
Abstract
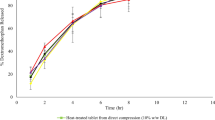
While polyethylene oxide (PEO)-based matrix tablets are frequently used as abuse-deterrent dosage forms, there is limited information available regarding how the selection of formulation components and manufacturing processes affect the resulting abuse-deterrent properties. The objective of the current study was to evaluate the effects of formulation and process variables on the abuse-deterrent features of PEO-containing tablets. Directly compressed tablets were prepared using three different PEO molecular weights (100,000; 900,000; and 5,000,000). As anticipated, sintering/thermal treatment above the melting point of PEO was crucial to impart crush-resistant features (tablet hardness > 500 N). In addition to the sintering temperature, the weight fraction of PEO in the tablets affected their mechanical strength, and at least 50% w/w PEO was required to impart the desired crush-resistant features. In addition, the formulation and process variables also impacted syringeability and injectability of the PEO gels formed when the tablets were hydrated to simulate attempted drug extraction. High molecular weight PEO (900,000 and 5,000,000) produced gels more resistant to syringeability and injectability compared to low molecular weight PEO (100,000). Sintering above the polymer melting point decreased PEO crystallinity after cooling, and longer sintering times resulted in PEO degradation producing lower viscosity gels with reduced resistance to syringeability and injectability. Although sintering above the melting point of PEO imparts optimal mechanical strength to the tablets, prolonged sintering durations negatively impact polymer stability and alter the resulting abuse-deterrent features of the PEO-based tablet formulations.



Similar content being viewed by others
References
Lessenger JE, Feinberg SD. Abuse of prescription and over-the-counter medications. J Am Board Fam Med. 2008;21(1):45–54. https://doi.org/10.3122/jabfm.2008.01.070071.
Commonly Abused Prescription Drugs. www.drugabuse.gov [11 Feb 2019]; Available from: https://www.drugabuse.gov/sites/default/files/rx_drugs_placemat_508c_10052011.pdf.
Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83:S4–7. https://doi.org/10.1016/j.drugalcdep.2005.10.020.
Understanding Abuse Deterrent Opioids. www.fda.gov [11 Feb 2019]; Available from: https://www.fda.gov/downloads/AboutFDA/WorkingatFDA/FellowshipInternshipGraduateFacultyPrograms/PharmacyStudentExperientialProgramCDER/UCM532123.pdf.
Muppalaneni S, Mastropietro DJ, Omidian H. Crush resistance and insufflation potential of poly (ethylene oxide)-based abuse deterrent formulations. Expert Opin Drug Deliv. 2016;13(10):1375–82. https://doi.org/10.1080/17425247.2016.1211638.
Royce AE. Directly compressible polyethylene oxide vehicle for preparing therapeutic dosage forms. United States Patent US 5,273-758; 1993.
Bartholomaeus JH, Arkenau-Marić E, Galia E. Opioid extended-release tablets with improved tamper-resistant properties. Expert Opin Drug Deliv. 2012;9(8):879–91.
Mastropietro DJ, Omidian H. Abuse-deterrent formulations: part 2: commercial products and proprietary technologies. Expert Opin Pharmacother. 2015;16(3):305–23. https://doi.org/10.1517/14656566.2014.970175.
Herry C, Monti A, Vauzelle-Kervroedan F, Oury P, Michel L. Reducing abuse of orally administered prescription opioids using formulation technologies. J Drug Deliv Sci Technol. 2013;23(2):103–10.
Webster LR, Markman J, Cone EJ, Niebler G. Current and future development of extended-release, abuse-deterrent opioid formulations in the United States. Postgrad Med J. 2017;129(1):102–10. https://doi.org/10.1080/00325481.2017.1268902.
Maincent J, Zhang F. Recent advances in abuse-deterrent technologies for the delivery of opioids. Int J Pharm. 2016;510(1):57–72. https://doi.org/10.1016/j.ijpharm.2016.06.012.
Ma L, Deng L, Chen J. Applications of poly (ethylene oxide) in controlled release tablet systems: a review. Drug Dev Ind Pharm. 2014(7):40, 845–851 https://doi-org.proxy.lib.uiowa.edu/10.3109/03639045.2013.831438.
Upadhye SB, Rajabi-Siahboomi AR. Properties and applications of polyethylene oxide and ethylcellulose for tamper resistance and controlled drug delivery. In: Repka MA, Langley N, DiNunzio J, editors. Melt extrusion: materials, technology and drug product design. New York: Springer New York; 2013. p. 145–58.
Rahman Z, Yang Y, Korang-Yeboah M, Siddiqui A, Xu X, Ashraf M, et al. Assessing impact of formulation and process variables on in-vitro performance of directly compressed abuse deterrent formulations. Int J Pharm. 2016;502(1–2):138–50. https://doi.org/10.1016/j.ijpharm.2016.02.029.
German RM. Sintering theory and practice. Hoboken: Wiley; 1996.
German RM. Liquid-phase sintering. Sintering Theory and Practice. Hoboken: Wiley; 1996. p. 225–307.
German RM. Solid-state sintering of mixed powders. Sintering Theory and Practice. Hoboken: Wiley; 1996. p. 178–221.
Singh R, Poddar SS, Chivate A. Sintering of wax for controlling release from pellets. AAPS PharmSciTech. 2007;8(3):E175–E83. https://doi.org/10.1208/pt0803074.
Cohen J, Siegel RA, Langer R. Sintering technique for the preparation of polymer matrices for the controlled release of macromolecules. J Pharm Sci. 1984;73(8):1034–7. https://doi.org/10.1002/jps.2600730805.
Nijenhuis A, Colstee E, Grijpma D, Pennings A. High molecular weight poly (L-lactide) and poly (ethylene oxide) blends: thermal characterization and physical properties. Polymer. 1996;37(26):5849–57. https://doi.org/10.1016/S0032-3861(96)00455-7.
Meruva S, Donovan MD. Effects of drug-polymer interactions on tablet properties during the development of abuse-deterrent dosage forms. AAPS PharmSciTech. 2019;20(3):93. https://doi.org/10.1208/s12249-018-1221-y.
Kinzler ER, Pantaleon C, Iverson MS, Aigner S. Syringeability of morphine ARER, a novel, abuse-deterrent, extended-release morphine formulation. Am J Drug Alcohol Abuse. 2019;45:1–8.
Ardizzone S, Dioguardi F, Mussini P, Mussini T, Rondinini S, Vercelli B, et al. Batch effects, water content and aqueous/organic solvent reactivity of microcrystalline cellulose samples. Int J Biol Macromol. 1999;26(4):269–77.
Rahman Z, Zidan AS, Korang-Yeboah M, Yang Y, Siddiqui A, Shakleya D, et al. Effects of excipients and curing process on the abuse deterrent properties of directly compressed tablets. Int J Pharm. 2017;517(1–2):303–11.
Yang L, Venkatesh G, Fassihi R. Characterization of compressibility and compactibility of poly (ethylene oxide) polymers for modified release application by compaction simulator. J Pharm Sci. 1996;85(10):1085–90.
Maggi L, Segale L, Torre M, Machiste EO, Conte U. Dissolution behaviour of hydrophilic matrix tablets containing two different polyethylene oxides (PEOs) for the controlled release of a water-soluble drug. Dimensionality study. Biomaterials. 2002;23(4):1113–9.
Nicholson LM, Whitley KS, Gates TS, Hinkley JA. Influence of molecular weight on the mechanical performance of a thermoplastic glassy polyimide. J Mater Sci. 2000;35(24):6111–21.
Boyce H. Mechanistic evaluation of polyethylene oxide for physical barrier type abuse deterrent formulations: techniques and in vitro methods [research]. College Park: University of Maryland; 2017.
Bartholomaus J, Schwier S, Brett M. New abuse deterrent formulation (ADF) technology for immediate-release opioids. Drug Dev Deliv. 2013;13(8):76–81.
Li L, Liu J-t, Ren Z-j, Yan S-k. Crystallization behavior of disproportionately high and low molecular weight PEO blends. Chin J Polym Sci. 2014;32(9):1199–209. https://doi.org/10.1007/s10118-014-1497-7.
Crowley MM, Zhang F, Koleng JJ, McGinity JW. Stability of polyethylene oxide in matrix tablets prepared by hot-melt extrusion. Biomaterials. 2002;(21):23, 4241–4228. https://doi.org/10.1016/S0142-9612(02)00187-4.
Huang F, Wei Q, Wang J, Cai Y, Huang Y. Effect of temperature on structure, morphology and crystallinity of PVDF nanofibers via electrospinning. e-Polymers. 2008;8(1).
Huang Z-M, Zhang Y-Z, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 2003;63(15):2223–53.
Ebagninin KW, Benchabane A, Bekkour K. Rheological characterization of poly (ethylene oxide) solutions of different molecular weights. J Colloid Interface Sci. 2009;336(1):360–7.
Liu W-H, Yu TL, Lin H-L. Shear thickening behavior of dilute poly (diallyl dimethyl ammonium chloride) aqueous solutions. Polymer. 2007;48(14):4152–65.
Acknowledgments
The authors would like to thank Dr. Ram Prakash Govindarajan for his assistance and contributions and Dr. Suresh Raghavan for his assistance and use of equipment for the syringeability and injectability studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meruva, S., Donovan, M.D. Polyethylene Oxide (PEO) Molecular Weight Effects on Abuse-Deterrent Properties of Matrix Tablets. AAPS PharmSciTech 21, 28 (2020). https://doi.org/10.1208/s12249-019-1565-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1565-y